Abstract
As the coronavirus disease (COVID-19) pandemic led to a global health crisis, there were limited treatment options and no prophylactic therapies for those exposed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Convalescent plasma is quick to implement, potentially provides benefits, and has a good safety profile. The therapeutic potential of COVID-19 convalescent plasma (CCP) is likely mediated by antibodies through direct viral neutralization and Fc-dependent functions such as a phagocytosis, complement activation, and antibody-dependent cellular cytotoxicity. In the United States, CCP became one of the most common treatments with more than a half million units transfused despite limited efficacy data. More than a dozen randomized trials now demonstrate that CCP does not provide benefit for those hospitalized with moderate to severe disease. However, similar to other passive antibody therapies, CCP is beneficial for early disease when provided to elderly outpatients within 72 hours after symptom onset. Only high-titer CCP should be transfused. CCP should also be considered for immunosuppressed patients with COVID-19. CCP collected in proximity, by time and location, to the patient may be more beneficial because of SARS-CoV-2 variants. Additional randomized trial data are still accruing and should be incorporated with other trial data to optimize CCP indications.
Introduction
As the coronavirus disease (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), led to a global health crisis in late 2019 and early 2020, there were limited treatment options and no prophylactic therapies for those who had been exposed to SARS-CoV-2. In this setting, COVID-19 convalescent plasma (CCP) was rapidly launched to address an emergent unmet medical need.
Convalescent plasma (CP) is one of the oldest therapies that continues to be used in emerging disease outbreaks, dating back to the 1890s for its first use to treat tetanus and diphtheria before the availability of antimicrobial therapy.1 A meta-analysis of more than 1700 patients with Spanish influenza pneumonia in 1918 demonstrated that those who received CP were significantly less likely to die, especially if the CP was transfused early.2 During the last century, CP has also been used to treat individuals infected with a multitude of viral infectious diseases including Argentine hemorrhagic fever, Ebola, Middle East respiratory syndrome (MERS), and SARS-CoV-1.3 In addition, CP has been used to prevent infection in those exposed but not yet infected (ie, postexposure prophylaxis) to polio, mumps, rabies, and hepatitis.3 Although the data are limited on use of convalescent plasma for respiratory viruses, it has been shown that CP is most effective when provided early in the disease course or as prophylaxis.4
CP is collected from individuals who were previously infected with the target virus and have high levels of antibodies. Transfusion of CP provides the susceptible recipient with passively transferred antibodies to the target virus, much like hyperimmune globulin or monoclonal antibody (mAb) administration. COVD-19 CP (CCP) is collected from individuals who had recovered from COVID-19. As CP has been used to treat other coronaviruses, it was hypothesized early in the COVID-19 pandemic that CCP may be effective as either postexposure prophylaxis and/or treatment of COVID-19.
Mechanism of action
CCP’s therapeutic potential is mediated through a variety of mechanisms. The neutralizing antibodies present in CCP can help to clear the virus.5,6 For CCP, it was demonstrated that most COVID-19 convalescent individuals develop a strong antibody response. However, the titers of neutralizing antibodies and their binding avidity are highly heterogeneous in CCP; approximately 20% of CCP units do not have detectable neutralizing antibody levels, and their levels varied by ABO blood group.6-9 Although CCP neutralizing titers generally correlate well with antibodies to the spike receptor binding domain (RBD), there is not a perfect correlation.7,10 There is a strong correlation between polyfunctional plasma and polyclonal antibody targeting of the SARS-CoV-2 spike protein peptides.7 Although it is unknown if it is beneficial, CCP also has significantly higher plasma levels of interferon-γ, Monocyte chemoattractant protein-1 (MCP-1), interleukin 10 (IL-10), IL-15, and IL-21 compared with plasma of healthy blood donors.11 In addition, it has been demonstrated that CCP contains antibodies that are able to mediate Fc-dependent functions, specifically phagocytosis, complement activation, and antibody-dependent cellular cytotoxicity against SARS-CoV-2.12 In the CONvalescent Plasma for Hospitalized Adults With COVID-19 Respiratory Illness (CONCOR-1) trial, the investigators demonstrated that higher levels of antibody-dependent cellular cytotoxicity and neutralizing titer were independently associated with improved outcomes.13 Thus, CCP likely provides benefits beyond only the neutralizing antibody.
Regulatory changes
The US Food and Drug Administration (FDA) regulatory framework evolved significantly during the pandemic. The FDA issued 8 guidelines for industry for investigational COVID-19 convalescent plasma, 5 emergency use authorization (EUA) files, and 3 recommendations for investigational CCP since 24 March 2020 until the last on 9 March 2021. These changes were driven by new data and testing abilities.
Initially, CCP was only administered under an emergency investigational new drug (eIND) application. Multiple clinical trials under INDs were also launched, and some are still enrolling (discussed below). In March 2020, the eIND was only recommended for patients with severe and life-threatening COVID-19. Because of significant pressure to allow easier access to CCP, the FDA established the Expanded Access Program (EAP) for participating institutions under one national IND approval and a master treatment protocol in April 2020. The EAP patient inclusion criteria were hospitalized patients with severe or life-threatening manifestations of COVID-19 or documented to be at high risk of developing such manifestations.14 Patients had to be at least 18 years old with confirmed laboratory diagnosis of COVID-19, who were admitted to an acute care facility and provided informed consent. Severe disease was defined as dyspnea, respiratory rate ≥ 30 breaths/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PPO2/FiO2) ≤ 300, and lung infiltrates > 50%; life-threatening disease was defined as respiratory failure, septic shock, or multiorgan failure. In August 2020, the FDA issued an EUA for CCP with an end date, which was extended, of administering CCP under the EAP ultimately of 31 May 2021. The EUA recommended use of a single unit of CCP (200 mL) administered early in the patient’s hospital course or to individuals with impaired humoral immunity.15
As regulatory updates were issued, CCP antibody level qualifications additionally changed. When CCP was initially collected in March 2020, the FDA guidance recommended antibody titer levels, if available. Although some organizations developed customized laboratory assays to measure antibody levels, most collection organizations lacked these assays. Once the EAP was launched, the FDA adjusted recommended antibody levels and requested retention samples for future titer testing. Notably, during this time, the demand for CCP greatly outweighed the supply; with no clinical data to demonstrate the benefit of high titers, many low-titer units were transfused to meet the demand. Eventually FDA-approved commercial antibody tests were used to distinguish and label high- or low-titer units, which were available through the EUA. As of February 2021, only high-titer CCP units are qualified for administration under the EUA.15
Donor eligibility also changed from requiring a documented negative COVID-19 polymerase chain reaction test to allowing donors who were 14 days after symptoms without needing a negative test. Initially, individuals previously with COVID-19 who were vaccinated were deferred, but this was eventually reversed. Another addition is that individuals who received monoclonal antibody therapy for COVID-19 are now deferred from donating CCP for 3 months.
Donation frequency is determined by the blood collection organization. Given that donor’s antibody levels fall over time and antibody levels are measured at each collection, once the antibody levels are below the organization’s cutoff levels, the donor is deferred from CCP donation.
Clinical evidence for CCP efficacy
As the COVID-19 pandemic began, more than 80 clinical trials of CCP were initiated to evaluate whether CCP was effective in different patient populations.4 The randomized trials assessed whether CCP would be effective in various patients, including (1) postexposure prophylaxis to prevent infection, (2) early treatment to prevent hospitalization, (3) treatment early in hospitalization for those requiring oxygen but not yet intubated (ie, Green Zone16), (4) severely ill or patients at high risk of becoming severely ill who are already intubated, and (5) pediatric patients. Although most of the clinical trials have completed enrollment, final data and publication are pending for the majority.
Sixteen peer-reviewed randomized controlled trials (RCTs) were included in this assessment of CCP’s efficacy (Table 1). These trials were heterogeneous in terms of location, alternate treatment availability, size, severity of illness in their patient cohort, timing of enrollment in relation to symptom onset, dose/antibody load of CCP used, and primary/secondary outcomes.17 This heterogeneity makes it difficult to compare trials; however, many of the conclusions drawn from the data point in a similar direction.
Using the World Health Organization (WHO) clinical progression scale for COVID-19,18 one can sort trials by the severity of COVID-19 in the targeted patient populations. Fourteen of the 16 trials enrolled patients with moderate to severe COVID-19.13,19-31 Of these, 12 trials encompassing more than 11 000 patients found no significant benefit of CCP compared with control for the various primary end points used.13,19-29 Although 2 smaller trials that enrolled a total of 111 patients with moderate to severe COVID-19 identified some potential benefits of CCP,30,31 the weight of evidence strongly points to the overall lack of CCP efficacy in patients with moderate to severe disease.
The overall lack of benefit with CCP may be explained by data from the Gharbharan trial, which found that most patients had high titers of autologous neutralizing antibodies against SARS-CoV-2 by day 10 after symptom onset; therefore, administration of high-titer CCP had no additional effect.22 In addition, most patients have entered the aviremic phase by day 10.32 Thus, CCP lacks a target for neutralization. However, trials of patients who are earlier in their disease course have shown mixed results.
The trial led by Libster et al33 enrolled elderly (either ≥75 years old or 65-74 years old with comorbidities) nonhospitalized patients who had experienced only mild COVID-19 symptoms for less than 72 hours. These patients were randomized to receive either 250 mL CCP or saline control. Libster et al33 found that significantly fewer patients receiving CCP met the primary endpoint of severe respiratory disease compared with the saline control group (16% CCP vs 31% control; risk ratio [RR], 0.52; 95% confidence interval [CI], 0.29-0.94; P = .03). Because the SARS-CoV-2 virus is no longer detectable in most patients by day 9,34 it is reasonable to conclude that this trial showed efficacy because patients were early in their illness and that the low levels of virus could be sufficiently neutralized by antibodies within the CCP. This concept is further supported by data from Libster et al33 that showed a correlation between risk reduction and the titer of the CCP unit transfused. While all patients in the CCP arm received high-titer CCP, those receiving the greatest antibody load had a risk reduction of 73.3% vs 31.4% for patients who received a lower concentration of antibody. These findings endorse the use of CCP early after infection while patients are still ambulatory. However, this was a small trial (N = 160) that did not reach its full enrollment goal and the only trial to explore CCP efficacy in this patient population.
The Convalescent Plasma in Outpatients with COVID-19 (C3PO) trial randomized patients who presented to emergency rooms in stable condition within 7 days of symptom onset (median symptom duration, 4 days).35 Enrolled patients were at least 50 years of age and had 1 or more risk factors for disease progression. The primary outcome was disease progression within 15 days of randomization. A total of 257 patients received 250 mL high-titer CCP and 254 received an equivalent volume of saline as a placebo. Disease progression was reported in 77 patients (30.0%) in the CCP arm and 81 patients (31.9%) in the placebo arm (risk difference, 1.9%; 95% CI, −6.0 to 9.8; posterior probability of superiority of CCP, 068). These findings were similar in post hoc subgroup analyses that looked at demographic characteristics, symptom duration, and risk factors. However, there were significantly more individuals who were hospitalized on the same day as the transfusion in the CCP arm compared with the control arm, suggesting an imbalance of the arms at the time of transfusion. The authors also note differences from the trial of Libster et al, including CCP was administered earlier in the trial of Libster et al (median time from symptom onset, 39.6 hours vs 4 days, Libster et al vs C3PO), and the patients were older (77.2 vs 51.6 years, Libster et al vs C3PO). C3PO closed early because of futility and did not reach its enrollment goal.
CCP dose
There is evidence that a high dose of neutralizing antibodies against SARS CoV-2 is critical for CCP to be effective36; however, the appropriate dose is unknown. Most trials delivered between 200 and 600 mL of high-titer CCP24,29,30,33 via 2 or more units of plasma,19,20,22,23,25,27,28,31 but there was intertrial variability with the antibody assays used and an inherent variability between units that cannot be controlled.
Confounding factors
Variants and CCP
As variants to the SARS-CoV-2 virus emerge, there is concern that the antibody profile in CCP must be specific for the variant of interest to benefit an infected patient. Live-virus neutralization assay shows that CCP from donors who had been infected against one strain of SARS CoV-2 was ineffective at neutralizing a variant strain.37 In addition, CCP that was against the wild-type virus showed fourfold lower neutralization capability when tested against the Delta variant.38 As a corollary to these findings, Kunze et al39 considered whether regional variation in the SARS-CoV-2 virus could be an important factor in CCP effectiveness. They tested the hypothesis that CCP is more effective when the CCP donor and treated patient were geographically close to one another. Using data from regional blood centers and a national registry of hospitalized patients with COVID-19, they compared the 30-day mortality rate for patients treated with “near sourced” CCP (≤150 miles) vs those receiving distantly sourced CCP (>150 miles). The mortality rate 30 days after transfusion for the full cohort was 9.76% (2728 of 27 952; 95% CI, 9.42%-10.11%). The cohort receiving near-sourced plasma had a lower death rate within 30 days (8.60%; 1125 of 13 088; 95% CI, 8.13%-9.09%) than the group receiving distantly sourced plasma (10.78%; 1603 of 14 864; 95% CI, 10.30%-11.29%; P < .001). The relative risk of death within 30 days in the group receiving near sourced CCP was lower than that seen for patients receiving distantly sourced CCP (relative risk, 0.80; 95% CI, 0.74-0.86). This study provided indirect evidence that the effectiveness of CCP was partly dependent on a match between the donor’s polyclonal antibodies and the patient’s virus. This information is critical for the appropriate assessment of clinical trial results, for guiding appropriate CCP use, and for the design and use of CCP stockpiles.
Immunosuppressed and CCP
Immunosuppressed individuals are at much higher risk of severe COVID-19 and have a poor humoral immune response to the mRNA SARS-CoV-2 vaccines and to the virus so they cannot clear the infection as well.40-43 Four key groups of immunosuppressed individuals include (1) patients with hematologic malignancy, (2) solid organ transplant recipients, (3) patients with rheumatologic issues, and (4) persons living with HIV.40 There were no randomized trials evaluating the use of CCP among immunosuppressed individuals. However, these individuals may benefit. In a case series of 17 patients with prolonged COVID-19 symptoms who were also immunosuppressed with anti-CD20 monoclonal antibodies, 16 patients (94%) significantly improved after transfusion of 4 units of CCP.44 This series, along with 75 case reports, case series, and 1 matched-control registry study representing more than 230 patients, found mortality was significantly lower and clinical improvement was more rapid among those individuals who received CCP.45
CCP collection and use
Beginning in March 2020, blood collectors and some hospital collection programs began collecting CCP.46 Different partnerships evolved to identify individuals who had COVID-19. These included blood collectors partnering with hospitals, public health organizations (local, state, and federal), and advocacy groups. One example is “The Fight is in US” coalition, which was a collaboration of public and private organizations, to attract CCP donors.
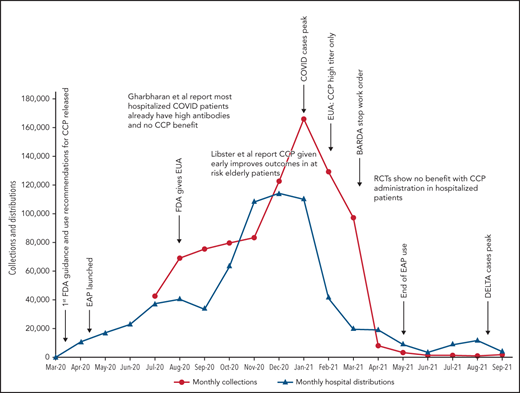
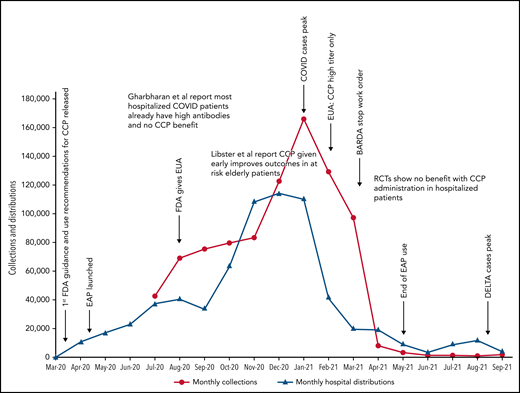
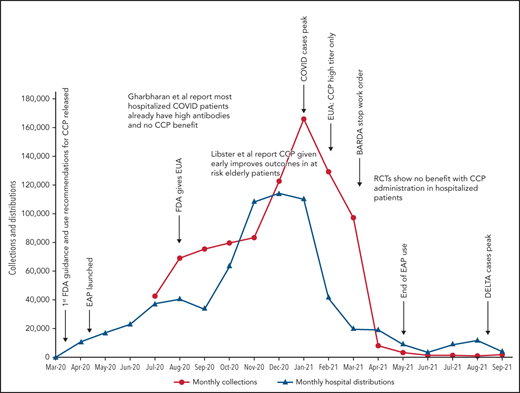
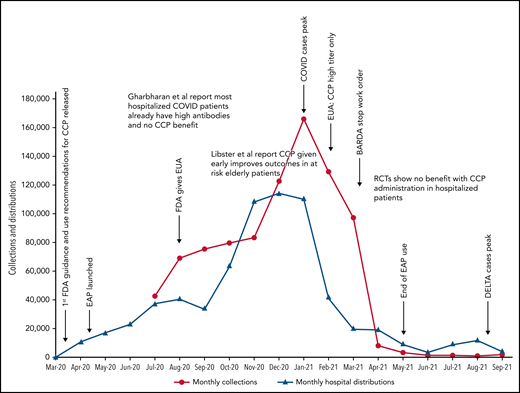
Because data for CCP’s efficacy were initially lacking, the Infectious Diseases Society of America and the National Institutes of Health recommended the use of CCP only in the setting of a clinical trial.47,48 Many countries used this approach, limiting CCP to trial settings.13,22,25,26 In the United States, however, the EAP and later EUA made CCP readily available to all hospitalized patients. Because early studies and trials had shown that CCP was as safe as conventional plasma (Table 2)49,50 and few other therapies were available, CCP became a popular choice for clinicians. Initially, the supply of CCP could not keep up with demand, as early in the pandemic, there were relatively few recovered patients available to donate, and blood centers were still ramping up efforts (Figure 1). To facilitate CCP’s availability, the US government provided funding through the Biomedical Advanced Research and Development Authority (BARDA), including collection startup costs. Initially, BARDA funding reimbursed only for units transfused; eventually, this was shifted to reimbursement for collections, which incentivized blood centers to increase supply. BARDA’s funding also removed the burden of payment from patients and their insurance, thus making CCP free for all recipients. This funding was available until 5 March 2021.51 As the supply of CCP rose, the demand also increased. In early 2021, the United States transfused approximately 25 000 units of CCP every week with more than 500 000 units transfused outside of clinical trials.
COVID-19 convalescent plasma collections and distributions to hospitals throughout the United States with key regulatory, pandemic, and publication dates. Data on monthly collections between March and May 2020 were not available. The raw data were provided by Jennifer Kapral and William Block of Blood Centers of America.
COVID-19 convalescent plasma collections and distributions to hospitals throughout the United States with key regulatory, pandemic, and publication dates. Data on monthly collections between March and May 2020 were not available. The raw data were provided by Jennifer Kapral and William Block of Blood Centers of America.
However, the use of CCP use did eventually shift and slow, likely because of the evidence from clinical trials. A weekly survey distributed by the AABB (Association for the Advancement of Blood & Biotherapies) showed that until May 2020, CCP was consistently prioritized for the patients with the most severe COVID-19.52 This trend changed between May and October 2020, with a decrease in use for severely ill recipients (from 52% to 37%) and a concomitant increase in CCP ordered for patients early in the course of disease (from 5% to 21%).4 The demand for CCP eventually waned just as BARDA funding ended and collections largely halted. As of this writing, there is a stockpile of more than 10 000 units of CCP in the United States, with approximately 2000 CCP units requested by hospitals per week (William Block, Blood Centers of America, written communication, October 2021).
The high demand also prompted the AABB to publish interim recommendations based on the data available in late 2020.49 The 5 recommendations from AABB state that (1) the risk of adverse events from CCP is low and comparable to standard plasma; (2) CCP is most effective when provide early in disease and likely not effective for those with late-stage disease; (3) high-titer CCP is most effective; (4) transfusion of out-of-group plasma is acceptable if your inventory is low; and (5) additional randomized controlled trial data are urgently needed to fully assess efficacy.
Although there has been substantial use of CCP in the United States, it has also been used globally. Most of the use of CCP in Canada and Europe was in clinical trials. As the COVID-19 pandemic spread, low- and middle-income countries (LMIC) looked to CCP as a potential therapy.53 The barriers that challenged LMIC were significant and similar to the problems faced with conventional blood collections: an overall blood deficit; dependence on paid and replacement donors; a limited capability for safe blood collection; fragmented infrastructure; and high rates of blood-borne infectious diseases.54,55 However, the cost of CCP is lower than other therapeutics such as monoclonal antibodies; thus, interest remained high in many countries. Efforts to export CCP from higher-income countries to LMICs faced barriers such as regulatory constraints, high costs for shipping, and supply issues.56 The International Society for Blood Transfusion developed an international multidisciplinary working group to develop guidance for the procurement and use of CCP by LMICs. This group noted that locally applicable strategies should be used to collect and produce CCP. One example is the use of whole blood donations for CCP rather than collecting plasma directly by apheresis. At least 2 countries that lacked apheresis equipment57 successfully deployed this approach.58,59 If the benefit of early CCP transfusions is confirmed, then additional measures will be necessary to promote the equitable distribution of CCP to areas of need.
Alternative immunotherapy products
In addition to CCP, other antibody therapeutics have been manufactured to treat patients with COVID-19. Rather than transfusing an entire unit of plasma, antibodies directed to SARS-CoV-2 can be pooled and purified from the plasma of recovered individuals to create hyperimmune globulin that delivers higher antibody doses and more diverse antibody targets in a smaller volume.60 In addition to purification of antibodies, monoclonal antibodies (mAbs) directed to SARS-CoV-2 have been created from single B-cell clones.61
The Inpatient Treatment with Anti-Coronavirus Immunoglobulin phase 3 clinical trial was a multicenter, double-blind placebo controlled randomized trial to assess the efficacy of remdesivir with anti-coronavirus hyperimmune intravenous immunoglobulin. The trial enrolled 600 adult patients who had been hospitalized for COVID-19 and had symptoms for 12 days or fewer. A press release stated the trial did not find efficacy,62 which is consistent with other study data that passive antibody therapy should be administered early in the disease course for optimal benefit, but final data are still pending.
Because of the demonstrated efficacy in patients with COVID-19 with early disease, mAbs have also been approved by the US FDA for EUA. All the mAbs for clinical use target the RBD of the spike glycoprotein. The mAbs neutralize the entry of SARS-CoV-2 entry into host cells by preventing the RBD from engaging with the target receptor angiotensin-converting enzyme 2. It is unknown if the mAbs also assist with humoral effector function. The 3 primary formulations of mAbs used in the United States include casirivimab-imdevimab (Regeneron), sotrovimab (Glaxo-Smith Kline), and bamianivimab-etesevimab (Eli Lilly). The clinical trials and the resulting EUA indication and National Institutes of Health treatment guidelines63 focused on outpatients, 12 years and older, with recently diagnosed mild or moderate COVID-19 symptoms and demonstrated that the mAbs decreased viral load and risk of hospitalization.65-66 The Infectious Disease Society of America recommends neutralizing mAbs for outpatients with mild to moderate COVID-19 who are at risk of progression to severe disease, but local variant susceptibility should be considered.67
There are advantages and limitations of CCP compared with either hyperimmune globulin or mAbs. The advantages are that CCP can be collected soon after individuals in the community are initially infected and identified. In contrast, hyperimmune globulin and mAbs take months or even years to purify, develop, and manufacture. In addition, CCP is polyclonal, so as the virus evolves and individuals develop a new antibody response, CCP continues to provide protection. Hyperimmune globulin is also polyclonal because it is manufactured from a pool of CCP. However, both the time and location of CCP collection compared with administration may influence efficacy because of variant changes. CCP is also much less expensive than the alternatives. However, the antibody levels in CCP are generally lower than those provided by hyperimmune globulin or mAbs. Hyperimmune globulin and mAbs may also be prepared for intramuscular administration, whereas CCP is intravenous. In addition, although CCP is safe and tested for the standard transfusion transmitted infections, hyperimmune globulin and mAbs have a higher safety profile because of using pathogen reduction and pooling techniques for hyperimmune globulin or the manufacturing techniques that do not involve human plasma for mAbs.
Conclusions
CCP was one of the most common treatments early in the COVID-19 pandemic. CCP provides antibodies that can directly neutralize the virus and Fc-dependent functions such as a phagocytosis, complement activation, and antibody-dependent cellular cytotoxicity against SARS-CoV-2. The early interest in CCP was tremendous, especially because there were limited treatments available for SARS-CoV-2. However, CCP was widely used before a coherent body of efficacy data were available.
The results of the currently available randomized trials suggest that high-titer CCP is efficacious only when given early after symptom onset (<72 hours) when the viral load is relatively low and before inflammatory damage is evident. The trial data strongly support the clinical futility of CCP after day 10, when patients have high autologous antibody titers, and the disease has entered the aviremic phase. Data from the mAb trials also support that antibody therapeutics are most efficacious when used early. Additional trials are necessary to better define the optimal time frame and dose at which high-titer CCP is effective.
The clinical trials were conducted in a dynamic setting with changes to clinical standard of care occurring during many of the trials. Therapies such as remdesivir, corticosteroids, toilizumab (mAb against IL-6 receptor-α), baricitinib (Janus kinase inhibitor), and prophylactic68,69 anticoagulation helped improve mortality but also made trial data more difficult to acquire and analyze. Most trials included a breakdown of the CCP vs control cohorts that showed whether the 2 arms were matched for select concomitant therapies.12,20,21,23-25,30 In some cases, trials included prespecified subgroup analyses to measure the effect of other therapies.24,25 Although it is impossible to control for all factors in a clinical trial, several trials attempted to consider these factors in their analyses. In addition, the quantities of the neutralizing antibody titers were not well characterized in the early use of CCP. As there is variability in CCP antibody titer, it may be that insufficient antibodies are provided in the doses that patients received.
Although much more data are currently available today compared with the beginning of the pandemic, there are still randomized trials evaluating CCP that are enrolling patients. Data on whether CCP is effective as postexposure prophylaxis and also outpatient treatments confirming the trial of Libster et al are anxiously awaited. However, currently, high-titer CCP should be prioritized for those with early disease, especially if they are immunosuppressed.
Acknowledgments
The authors thank Bill Block, Jennifer Kapral, and Joshua Xianming Zhu for assistance with Figure 1.
Authorship
Contribution: C.S.C., A.A.R.T., and B.H.S. wrote the article and created the figure and tables.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Beth H. Shaz, Department of Pathology, Duke University, 2400 Pratt St, Room 9011, Durham, NC 27705; e-mail: beth.shaz@duke.edu.