Abstract
Light-chain amyloidosis has come far, with the first treatment getting regulatory approval in 2021. Daratumumab-based regimens achieve deep hematologic and organ responses, offering a new therapeutic backbone. Early identification, correct fibril typing, challenges of the very advanced patient, and lack of therapies to remove amyloid deposits remain under study, but are, as yet, elusive. We review the progress of treatment in AL amyloidosis, the impact of daratumumab, and the next steps after treatment.
Introduction
Systemic AL amyloidosis is an intriguing, complex, multisystem disease that is challenging for physicians from suspicion of diagnosis to management.1 Unstable circulating monoclonal light chains originating from a plasma cell or a B-cell clone cause direct tissue proteotoxicity from prefibrillar aggregates and oligomers that form proteolysis-resistant tissue fibrils, a duo causing rapidly progressive organ dysfunction and death. The silent start, multiple organ targets, and rapid decline form a devastating combination that has defied attempts at early recognition and effective treatment. Welcome winds of change have come with novel antiplasma cell therapies that have progressively improved survival in the past decade, attracting the attention of researchers and industry to this previously orphan disease, culminating in 2021 with the licensing of daratumumab in combination with cyclophosphamide, bortezomib, and dexamethasone (dara-VCd) for patients with newly diagnosed AL amyloidosis.2
The 3 key elements in the management of AL amyloidosis are correct, early recognition of the diagnosis; rapid control of the amyloidogenic light chains; and improvement of the function of the end organs damaged by the amyloid deposits: of those, the first and last remain unmet medical needs.
Evolution in the treatment of AL amyloidosis
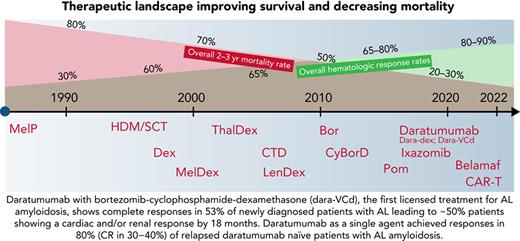
The major changes in the steps for treating AL amyloidosis (Figure 1) started with demonstration of the positive survival impact of high-dose melphalan and autologous stem cell transplantation (HDM/SCT) in the mid-1990s making it, to date, an important standard of care in select patients with early disease.3 Stringent selection criteria have reduced treatment-related mortality to <5%. The key advantage of HDM/SCT is a prolonged duration of hematologic complete response (CR), event-free survival, and greater than a decade overall survival (OS) in patients achieving a CR.4
The changing landscape in AL amyloidosis. The number of available therapies has improved OS in the past 2 decades from a median of ∼1 year to more than 5 years, mirrored by a significant decline in mortality in all stages, except in cases of very advanced cardiac disease.
The changing landscape in AL amyloidosis. The number of available therapies has improved OS in the past 2 decades from a median of ∼1 year to more than 5 years, mirrored by a significant decline in mortality in all stages, except in cases of very advanced cardiac disease.
The prolonged survival with the use of oral melphalan with dexamethasone was the change in the next step, leading to its adoption as a standard of care in non–transplant-eligible patients in the mid-2000s.5 Buoyed by the success of immunomodulator agents in multiple myeloma, trials with thalidomide6 and lenalidomide, with or without additional alkylators in AL,7 showed adequate (but rarely deep) responses, with surprising and unexplained intolerance (fatigue, renal impairment, and an increase in cardiac biomarkers).
Bortezomib was the third and key game changer. Marked excess of misfolded toxic light chains in AL cause the plasma cells to be a log more sensitive to proteasome inhibition in AL than in multiple myeloma.8 It was “reasonably” well tolerated, and CRs were seen in the relapsed setting,9 especially with combination of bortezomib-dexamethasone with cyclophosphamide (VCD).10,11 In the front-line setting, very good partial response or better has been seen in more than half of all patients, with CRs in a quarter of the patients treated with VCD or VMdex.12,13
Progressive and steady improvement in survival in AL amyloidosis can be well tracked to the described treatment landmarks,14-16 but challenges remain. Patients with advanced cardiac disease (N-terminal–pro B-type natriuretic peptide [NT-proBNP], >8500 pg/mL) continue to have high early mortality and morbidity with multiple hospital admissions; a glimmer of hope is seen in patients achieving CR (a small proportion) with better long-term outcomes. The recognized cardiac toxicity of proteasome inhibitors leads to concern about contribution of therapy to early deaths in AL17 despite the lack of clear trends in case-control data.18 Crucially, the improvement in organ function is slow and limited (∼20% patients at 6-12 months).
Two other key findings intensified the need to find novel combinations: (1) significant survival benefit of achieving a very deep light-chain response over and above CR (difference in the involved and uninvolved free light chains [dFLCs] <10 mg/L12 or involved FLC [iFLC] <20 mg/L)19,20 and minimal residual disease (MRD) assessment showing that MRD negativity leads to organ responses in >75% of cases.21,22 (2) Demonstration that a rapid response breaks the fibrillogenesis-proteotoxicity chain, stopping or slowing organ failure and translating into better outcomes; it is now is clear that deep response at 1 month is a crucial marker (where daratumumab has a major role).23
Daratumumab in AL amyloidosis
Daratumumab is a high-affinity human IgGκ1 monoclonal antibody that binds to CD38, an antigen ubiquitously expressed all plasma cells, causing cell death by multiple pathways. Daratumumab containing triplet and quadruplet combinations in myeloma can lead to deep MRD-negative responses with improved PFS and OS.
Daratumumab in relapsed AL amyloidosis
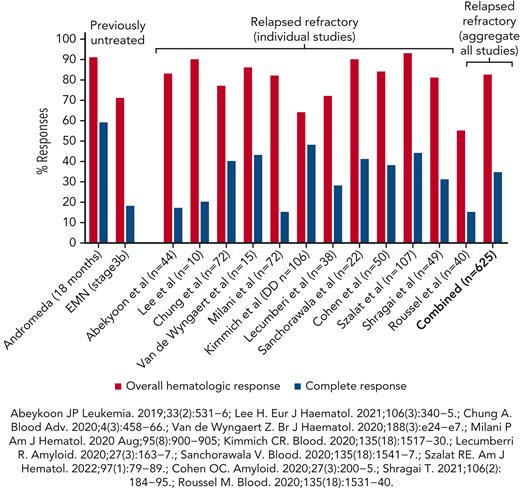
Single-agent daratumumab was reported to be effective in relapsed AL amyloidosis in 2 cases,23 followed by a large retrospective study of 25 patients showing rapid hematologic responses (CR, 36%, and very god partial response [VGPR], 24%).24 More than 12 studies have been published in which daratumumab was used in a total of 625 patients with relapsed AL amyloidosis (2 prospective phase 2 studies and the rest retrospective; Table 1)25-36 and showed a combined overall hematologic response rate of 83% (Figure 2). Two prospective phase 2 trials in relapsed AL amyloidosis confirmed these findings of high VGPR or better in 48% to 86% with a median time response of 1 to 4 weeks,33,37 translating to improved organ function with renal and cardiac responses in more than one-half of all the patients treated. However, the CRs are seen in only approximately one-third of the patients (variable proportion in individual studies reflecting the impact of prior therapies and patient selection). Long-term follow-up of patients from Boston University (BU)35 showed that those continuing to receive daratumumab for >12 cycles had significantly longer major organ deterioration-progression-free survival (MOD-PFS; 30 vs 13 months; P = .0018) and OS (not reached vs 15 months; P < .0001). NT-proBNP >8500 pg/mL, presence of 1q21 gain, and shorter duration of therapy (≤12 cycles) were strong negative predictive factors for outcomes of daratumumab therapy in AL amyloidosis.35 In a study by a German group, cardiac responses were seen in 22% with daratumumab-dexamethasone and 26% with additional bortezomib.31 Nephrotic range proteinuria was associated with poorer event-free survival and OS32 but not in the recently updated series from our group in BU,35 potential urinary loss of daratumumab in nephrotic patients compromising responses needs further clarification as the pharmacokinetic data from the ANDROMEDA (broadly similar PKs in AL amyloidosis and myeloma) did not model patients with nephrotic vs nonnephrotic.38
OS and hematologic CR to daratumumab-based therapies in newly diagnosed and relapsed/refractory systemic AL amyloidosis.
OS and hematologic CR to daratumumab-based therapies in newly diagnosed and relapsed/refractory systemic AL amyloidosis.
Daratumumab in front-line treatment of AL amyloidosis
ANDROMEDA was the pivotal phase 3 trial comparing dara-VCd (up to 24 cycles) with VCd alone (6 cycles) in 388 patients with newly diagnosed systemic AL amyloidosis2 (excluding very advanced disease), with a primary end point of hematologic CR and secondary end points of organ response and MOD-PFS. The December 2021 update,38 at a median follow-up of 25.8 months, reports that hematologic CR and VGPR were significantly superior for the dara-VCd arm compared with VCd alone (59.5% vs 19.2% and 79.0% vs 50.3%, respectively) with significantly better MOD-PFS in the daratumumab group. The hematologic response in the dara-VCd arm were rapid compared with that in the VCd arm (median time to first response, 16 days vs 24 days, respectively). At 18 months, cardiac and renal responses were also higher in the dara-VCd arm (53% and 58%, respectively) compared with the VCd arm (24% and 26%, respectively). A total of 79 deaths have occurred (dara-VCd, 34 patients,17%; compared with VCd, 45 patients, 24%) but survival data are still not mature.
The European Myeloma Network reported early results of a phase 2 study of stage IIIb cardiac AL amyloidosis in 17 patients (planned recruitment, 40 patients) with an overall response rate of 71% (3 patients [18%] achieving CR, 6 [35%] VGPR, and 3 [18%] a PR) and OS of 70% and 53% at 6 and 12 months, respectively.39
Ongoing trials include combinations of daratumumab with ixazomib (newly diagnosed; www.clinicaltrials.gov, registered as NCT03283917), with pomalidomide (relapsed; NCT04895917), and with daratumumab, bortezomib, and dexamethasone in advanced cardiac AL (newly diagnosed; NCT04474938).
Toxicity of daratumumab in AL amyloidosis
Overall, in AL amyloidosis, apart from infections, the toxicity of daratumumab appears to be limited with only rare grade 3 or 4 infusion or administration-related reactions. In the ANDROMEDA trial, serious adverse events (SAEs) occurred in 43% vs 34% in the dara-VCd vs VCd groups, with slightly higher incidence of grade 3 or 4 infections (16.6% vs 10.1%, respectively), but led to treatment discontinuation in only ∼4% of patients in either group. Lymphopenia, neutropenia, and respiratory infections were the commonest grade ≥3 AEs.2 Heart failure was reported in 6.2% of the daratumumab group vs 4.8% in the control group. In relapsed AL amyloidosis, infections occurred in ∼60% patients with about one-third being grade 3 or higher.29 In the BU study, atrial fibrillation and heart failure were reported in 18% and 14%, respectively.33 In patients with stage IIIb AL amyloidosis, there were 6 deaths, 65% patients had SAEs, and 9 (53%) had cardiac SAEs.39 All SAEs and deaths were considered to be unrelated to daratumumab.
Limitations
Data on daratumumab in AL is rapidly accumulating but many limitations remain. Until the European Myeloma Network study shows the impact of daratumumab in stage IIIb cardiac AL amyloidosis, the data will remain unclear. The impact and safety of dara-VCd in advanced cardiac AL amyloidosis is in the early stages of study in China. Dara-VCd, while moving the care of patients with AL amyloidosis significantly forward, still involves the components (bortezomib, dexamethasone) that cause significant clinical problems. A lack of dramatic reduction in early mortality in ANDROMEDA, despite the remarkable rapidity of hematologic response, raises a crucial question: have we reached the limits of what can be achieved by simply reducing the precursor without addressing the actual deposits? Last, with dara-VCd, 40% of patients did not achieve a CR. Strategies for improving responses, in those patients and in those having a relapse after dara-VCd, remain unclear.
The impact of maintenance daratumumab in AL amyloidosis is not clear because ANDROMEDA had no maintenance randomization, but data from BU show that daratumumab for >12 cycles leads to better MOD-PFS and OS. Data from the United Kingdom have demonstrated that patients treated with VCD alone without maintenance and reaching a CR had not reached median time to next treatment at 4 years. Data on benefit, safety, and cost effectiveness (a key requirement in many health care systems) of ongoing maintenance are crucially needed.
Other considerations in treatment of AL amyloidosis
The impact of the underlying clonal disease needs greater focus. Patients with plasma cell burden >20%40 and/or presenting with high FLC (>400 mg/L) have a high risk of early relapse and substantially worse outcomes.40 Patients with t(11;14) translocation (40% of patients) have poorer responses and worse outcomes with bortezomib-based therapies,41 an adverse prognostic factor potentially overcome by dara-VCD, as seen in subgroup analysis of ANDROMEDA. Importantly, deep responses can be reached in ∼70% of this group with venetoclax with low toxicity.42 Shifting the focus toward the use of clonal parameters by using targeted therapy and patient selection for escalation or deescalation of therapy in patients with high or low clonal burden, respectively, is needed. The exciting data from active immunotherapy (chimeric antigen receptor T cells and bispecific antibodies) in relapsed myeloma, where responses are seen in hours to days,42 could be truly transformational for AL amyloidosis with even the prospect of a “cure” because of the monoclonal-gammopathy-of-undetermined-significance nature of the clone in the majority.43
Last, the 2 critical and ultimate therapeutic goals are rapid improvement in organ function and impact of therapies on quality of life. Quality-of-life studies remain small, and data are limited. CAEL101 (an antifibril antibody) showed encouraging renal and cardiac responses in a phase 1 trial44; phase 3 studies are ongoing (NCT04512235 and NCT04504825). With a survival benefit in post hoc analysis of the VITAL trial, prospective reappraisal of birtamimab is in progress (NCT04973137).44 The tools to assess the impact of therapies that remove amyloid fibrils are still a missing ingredient because of the lack of clarity regarding the utility of the current response criteria in this setting. Evaluation of new tools, such as pan amyloid imaging agents AT0145 or florbetaben/florbetapir46; target engagement demonstration using fluorodeoxyglucose-positron emission tomography or macrophage-specific markers46; reevaluation of the role of cardiac biomarkers (NT-proBNP/troponin); and developing amyloidosis-specific patient-reported outcome measures PROMS (patient reported outcome measures) is imperative. There must be a wider appreciation that therapies for the effects on other organs that have a severe impact on quality of life and survival (advanced renal dysfunction, severe autonomic neuropathy, gastrointestinal symptoms, and the ubiquitous fatigue) are needed. The time is approaching for reappraisal of the role of SCT in AL amyloidosis with unprecedented hematologic responses rated with incorporation of dara-VCd in the AL treatment paradigm.
Conclusions
AL amyloidosis has entered a new and exciting phase. Questions and challenges for the patients with very advanced disease remain, but treatment with daratumumab-based regimens clearly offers the chance to reach deep responses in the remaining 70% to 80% of patients with less-advanced disease, translating into organ responses and improved quality of life and, most likely, better OS. This is a welcome broad brush across the board. Early identification remains elusive as ever and efforts need be redoubled. The increasing identification of transthyretin amyloidosis in older patients with an overlapping presence of monoclonal gammopathy of undetermined significance makes correct typing of the pathologic amyloid fibrils truly critical. We must embark on the subtler steps: refinement of therapies based on biomarkers, clonal characteristics, and genetics; addressing the question of organ improvement; resolving the issue of ongoing maintenance; capturing cost and quality impacts of therapy; incorporating therapies that remove amyloid fibrils and thus accelerating organ improvement; and using the upcoming wave of active immunotherapy toward a curative approach.
Is daratumumab a small step or giant leap? We say a welcome giant leap without a doubt; but remember, it is only the first leap, and we have a way to go.
Authorship
Contribution: A.D.W. and V.S. conceived and wrote the manuscript; and both authors approved the final draft of the manuscript.
Conflict-of-interest disclosure: A.D.W. has received honoraria and research funding from and has served on advisory boards of Janssen, Caelum, Prothena, Alexion, Celgene (BMS), Glaxo SmithKline (GSK), Amgen, and Takeda. V.S. has received honoraria and research funding from and has served on advisory boards of AbbVie, Takeda, Janssen, Prothena, Celgene, Caelum, Regeneron, Proclara, Oncopeptide, Kyropharm, and Sorrento.
Correspondence: Ashutosh Wechalekar, National Amyloidosis Centre, University College London (Royal Free Campus), Rowland Hill St, London NW3 2 PF, United Kingdom; e-mail: a.wechalekar@ucl.ac.uk.