Key Points
Donor NK cells trigger recipient dendritic cells to synthesize B2M, which stimulates cKIT-L and interleukin-7 production.
Adoptive transfer of ex vivo–expanded donor alloreactive NK cells accelerates post–bone marrow transplant immune reconstitution.
Abstract
Allogeneic hematopoietic transplantation is a powerful treatment for hematologic malignancies. Posttransplant immune incompetence exposes patients to disease relapse and infections. We previously demonstrated that donor alloreactive natural killer (NK) cells ablate recipient hematopoietic targets, including leukemia. Here, in murine models, we show that infusion of donor alloreactive NK cells triggers recipient dendritic cells (DCs) to synthesize β-2-microglobulin (B2M) that elicits the release of c-KIT ligand and interleukin-7 that greatly accelerate posttransplant immune reconstitution. An identical chain of events was reproduced by infusing supernatants of alloreactive NK/DC cocultures. Similarly, human alloreactive NK cells triggered human DCs to synthesize B2M that induced interleukin-7 production by thymic epithelial cells and thereby supported thymocyte cellularity in vitro. Chromatography fractionation of murine and human alloreactive NK/DC coculture supernatants identified a protein with molecular weight and isoelectric point of B2M, and mass spectrometry identified amino acid sequences specific of B2M. Anti-B2M antibody depletion of NK/DC coculture supernatants abrogated their immune-rebuilding effect. B2M knock-out mice were unable to undergo accelerated immune reconstitution, but infusion of (wild-type) NK/DC coculture supernatants restored their ability to undergo accelerated immune reconstitution. Similarly, silencing the B2M gene in human DCs, before coculture with alloreactive NK cells, prevented the increase in thymocyte cellularity in vitro. Finally, human recombinant B2M increased thymocyte cellularity in a thymic epithelial cells/thymocyte culture system. Our studies uncover a novel therapeutic principle for treating posttransplant immune incompetence and suggest that, upon its translation to the clinic, patients may benefit from adoptive transfer of large numbers of cytokine-activated, ex vivo–expanded donor alloreactive NK cells.
Introduction
Allogeneic hematopoietic cell transplantation is the most powerful therapy for high-risk leukemia.1,2 Unresolved issues are leukemia relapse, graft-versus-host disease, and prolonged immune incompetence. In pioneering major histocompatibility complex (MHC) haplotype-mismatched (“haploidentical”) transplantation,3 we discovered that donor-versus-recipient natural killer (NK) cell alloreactions play a beneficial role.4,5 Human NK cell function is finely tuned by clonally distributed cell surface receptors, including inhibitory receptors termed “killer-cell immunoglobulin-like receptors” (KIRs), that recognize human leukocyte antigen (HLA) class I allele groups (“KIR ligands”), such as Bw4, C1, and C2.4-13 In transplants that are KIR ligand-mismatched in the donor-versus-recipient direction, certain NK cells in the donor repertoire express KIR(s) for HLA class-I allele group(s) present in the donor but absent in the recipient. Such cells sense the missing expression of the inhibitory self HLA class-I KIR ligand and are activated to kill recipient targets. NK cell alloreactions reduce leukemia relapse and improve survival.4,5,13 In murine F1 H-2d/b→parent H-2b transplants or in H-2d→H-2b transplants, donor NK cells that do not express the H-2b–specific Ly49C/I inhibitory receptor (but instead bear H-2d–specific Ly49A/G2 receptors) cannot be blocked by the mismatched recipient MHC haplotype and are activated to kill the recipient’s targets.4,5 In this model, the pretransplant infusion of donor-versus-recipient alloreactive NK cells ablates leukemic cells, recipient T cells responsible for graft rejection, and recipient dendritic cells (DCs), that trigger graft-versus-host disease.4,5
Here, in murine bone marrow transplant (BMT) models and in a human cell culture system, we show that donor-versus-recipient alloreactive NK cells triggered recipient DCs to synthesize a protein that played a key role in stimulating production of 2 master regulators of lymphocyte development, interleukin-7 (IL-7) and cKIT ligand (cKIT-L), that greatly accelerated post-BMT recovery of donor-derived B and T lineage cells and DCs. Proteomics analyses demonstrated the molecule produced by DCs to be β-2-microglobulin (B2M). Genetics analyses strengthened this finding and showed the in vivo role of B2M in immune reconstitution.
Materials and methods
Murine bone marrow transplants
Experiments were performed in accordance with the Italian Ethics Approval Document for Animal Experimentation. Initial experiments investigated the effects of alloreactive NK cell infusions on post-BMT immune reconstitution. Six- to 8-week-old female C57BL/6 (H-2b) mice (Charles River Laboratories, Calco, Italy) were conditioned with lethal total-body irradiation (TBI) (8 Gy). One day later, mice received an IV infusion (through the tail vein) of alloreactive NK cells from 6- to 8-week-old female Balb/c (H-2d) mice (Charles River Laboratories). Control mice were given nonalloreactive (ie, syngeneic) NK cells. NK cells were obtained from splenocytes by Ficoll-Hypaque gradient centrifugation and immunomagnetic selection using a mouse NK cell isolation kit (Miltenyi, Bergisch Gladbach, Germany). Before infusion, NK cells were cultured for 4 days in the presence of 2000 IU/mL human IL-2 (Miltenyi) at the concentration of 2 × 106 cells/mL in RPMI culture medium supplemented with 10% fetal calf serum (Invitrogen, CA). DX5 antibody staining of murine NK cell–specific α2 integrin showed ≥98% purity of ex vivo–expanded, IL2-activated NK cells used for infusions in bone marrow–transplanted mice (DX5− cells were CD3+ T cells) (supplemental Figure 1). Before infusion, NK cells were washed. NK cell infusions contained 106 alloreactive NK cells in a final volume of 500 μL phosphate-buffered saline. NK cells were analyzed for expression of inhibitory receptors for MHC class I by fluorescein isothiocyanate–conjugated anti-Ly49A, fluorescein isothiocyanate–conjugated anti-Ly49G2, and phycoerythrin-conjugated anti-Ly49C/I monoclonal antibodies (BD Biosciences, CA). Multicolor immunofluorescence was analyzed by flow cytometry using a 2-laser FACScanto (BD Biosciences). Such an analysis demonstrated that the NK cells from H-2d donor mice contained a population (∼40%) that did not carry the H-2b–specific Ly49C/I inhibitory receptor and, consequently, was potentially alloreactive against recipient H-2b targets. Alloreactivity was tested using H-2b mouse concanavalin A (Sigma-Aldrich, Missouri) T-cell blasts as targets in a 51Cr-release cytotoxicity assay.4 One day after the NK cell infusion, mice received 10 × 106 T-cell–depleted bone marrow (BM) cells collected by flushing the femur and tibia shafts of H-2d mice. BM cells were T-cell–depleted by negative immunomagnetic selection using anti-CD5 microbeads (Miltenyi) as previously described.4
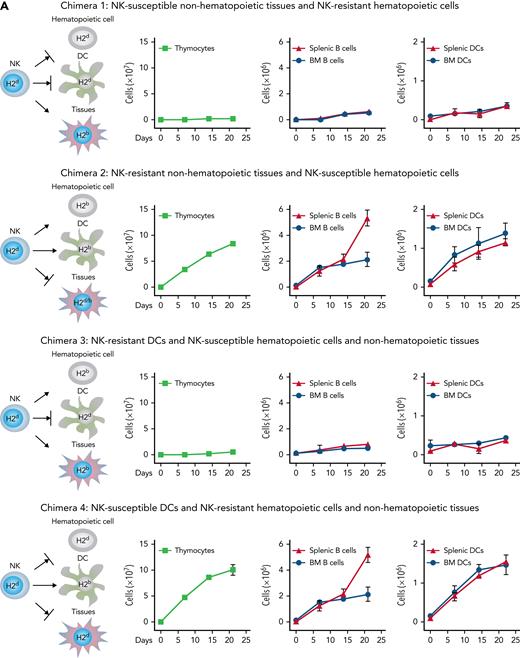
In order to investigate whether a specific recipient cell type triggered alloreactive NK cells to accelerate post-BMT immune reconstitution, 4 types of recipient chimeras were constructed in which hematopoietic and nonhematopoietic tissues differed in their MHC class I types so as to make tissues potentially susceptible (H-2b) or resistant (H-2d or H-2d/b) to alloreactivity mediated by NK cells from the H-2d donor mouse. Chimera 1 displayed NK-susceptible nonhematopoietic tissues and NK-resistant hematopoietic cells. Chimera 2 displayed NK-resistant nonhematopoietic tissues and NK-susceptible hematopoietic cells. Chimera 3 displayed DCs that were potentially susceptible to donor alloreactive NK cells, whereas all other recipient hematopoietic and nonhematopoietic cells were resistant. Chimera 4 displayed NK-resistant DCs and NK-susceptible hematopoietic and nonhematopoietic cells. BM graft and NK cell numbers were the same as in the transplants described above. For a detailed description, see “Construction of transplantation chimeras” in supplemental Methodology in supplemental Material (available on the Blood Web site).
In a further series of experiments, mice received an infusion of supernatants obtained from NK/DC cocultures. Such experiments were designed to investigate whether soluble factor(s) contained in alloreactive NK/DC coculture supernatants mediated biological effects. Therefore, these experiments were intentionally performed using either allogeneic or syngeneic donor-recipient transplant pairs, with identical results. The details of such experiments are extensively outlined under “In-vivo infusions of NK/DC coculture supernatants” in supplemental Methodology in supplemental Material.
Statistical analysis
For statistical analyses, see supplemental Methodology.
Immune reconstitution
Immune reconstitution was evaluated by multicolor immune-fluorescence, enzyme-linked immunosorbent assay, and quantitative polymerase chain reaction (qPCR). For a detailed description of methodologies, see “Immune reconstitution” in supplemental Methodology in supplemental Material.
Human cell cultures
For methodologies dealing with cloning of human alloreactive NK and T cells, cytotoxicity assays against allogeneic DCs,13 generation of NK/DC coculture supernatants, and the human thymic epithelial cells (TEC)/thymocyte culture system,14 see “Human cell cultures” in supplemental Methodology in supplemental Material.
Proteomics
The biochemical analyses were designed to identify a newly synthesized, biologically active protein (“the immune rebuilding factor”) and to define its biochemical features. In order to biochemically define the “immune rebuilding factor,” NK/DC coculture supernatants were subjected to hydrophobicity chromatography (HIC), reverse phase (RP) chromatography, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and autoradiography. SDS-PAGE bands displaying immune rebuilding activity were excised and subjected to protein digestion and peptide extraction before nano–liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis. For the detailed description of such experiments, see “Proteomics” in supplemental Methodology in supplemental Material.
Analysis of the role of the B2M gene
In order to document the role of B2M in accelerating posttransplant immune reconstitution, supernatants obtained from cocultures of alloreactive NK-DCs or nonalloreactive NK/DC culture combinations were B2M immunodepleted using the polyclonal rabbit anti-B2M FL-119 Ab (Santa Cruz Biothecnology), followed by a secondary goat anti-rabbit Ab subsequently adsorbed on a Sepharose column. A non-B2M immune rabbit serum was used as a negative control. In order to obtain genetic evidence for B2M’s role in accelerating immune reconstitution, the following experiments were performed: (1) B2M-knockout (KO) H-2b mice (Jackson Laboratories) were lethally irradiated and were given an infusion of 106 alloreactive wild-type (WT) H-2d NK cells before T-cell–depleted BMT (10 × 106 cells) from WT H-2d mice. (2) Lethally irradiated WT H-2b mice were given an infusion of 500 μL supernatant from cocultures of 5 × 106 alloreactive WT H-2d NK cells and 5 × 106 DCs from B2M-KO H-2b mice. (3) B2M-KO mice received an infusion of supernatants obtained from cocultures of alloreactive WT H-2d NK cells and WT H-2b DCs. In order to obtain genetic evidence of the B2M’s role in the human system, the B2M gene was silenced in human DCs. Human DCs were transfected using Amaxa P3 Primary Cell 4D-Nucleofector X Unit (Lonza, Basel, Switzerland) in accordance with the manufacturer’s instructions. Aliquots of 2 × 106 DCs were electroporated with 20 pmol of B2M-specific or scramble short interfering-RNA, resuspended in cell culture medium, and incubated overnight at 37°C/5% CO2. B2M-silenced DCs or DCs electroporated with scramble RNA or untreated DCs were cocultured with alloreactive NK cell clones. NK/DC coculture supernatants were added to the human TEC/thymocyte culture.
Gene expression profiling
In order to obtain information about the downstream pathways triggered by B2M on stromal cells, B2M KO mice were used in order to avoid any interference by endogenous B2M. They were lethally irradiated, and 7 days later, they were infused with supernatants obtained from cocultures of alloreactive WT H-2d NK cells and WT H-2b DCs or nonalloreactive (syngeneic) NK/DC coculture combinations. After 12 hours, RNA from murine BM was subjected to gene expression profiling. For the detailed description of such experiments see, “Gene expression profiling” in supplemental Methodology in supplemental Material.
Results
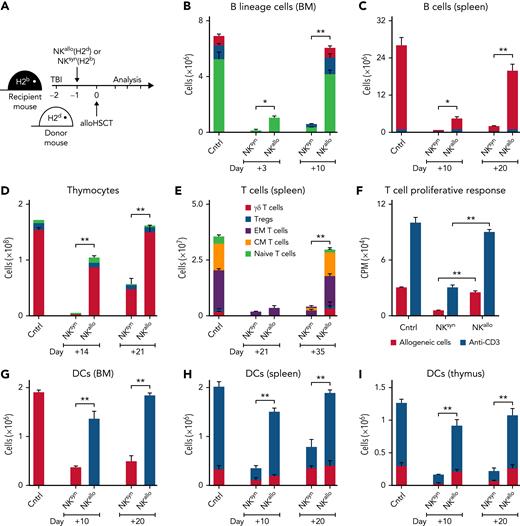
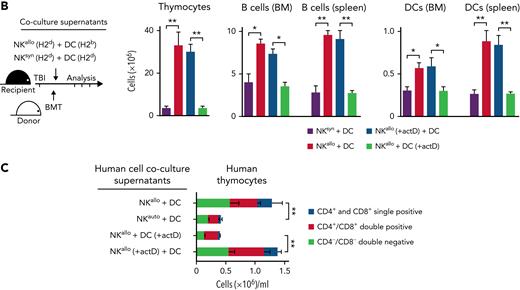
H-2b recipient mice were conditioned with TBI and received an infusion of IL-2–activated NK cells from H-2d mice. Such NK cells contain a population that lacks the H-2b–specific Ly49C/I inhibitory receptor and, consequently, is alloreactive against H-2b lympho-hematopoietic cells.4 Subsequently, mice were transplanted with extensively T-cell–depleted BM from H-2d mice (contaminating T cells in the BM graft were <0.2%) (Figure 1). The engrafted hematopoietic stem cells quickly gave rise to a greatly accelerated recovery of major players of the immune system, such as developing and mature T and B cells, as well as of DCs in the thymus, BM, and spleen, which quickly reached values comparable with those of donor mice (see Figure 1 and its legend for a detailed description of the various developing T, B, and DC subsets). We next investigated whether interactions between donor NK cells and specific recipient cell types were responsible for accelerated post-BMT immune reconstitution. Because alloreactive NK cells are preferentially activated by hematopoietic cells,4 we constructed recipient chimeras in which hematopoietic lineage cells and nonhematopoietic tissues differed in their MHC class I types so as to make tissues potentially susceptible (H-2b) or resistant (H-2d or H-2d/b) to alloreactivity mediated by donor H-2d NK cells (Figure 2A). Recipient chimeras were lethally irradiated, received an infusion of H-2d NK cells, and subsequently received a T-cell–depleted BMT from the H-2d donor mice. In chimeras with NK-susceptible nonhematopoietic tissues and NK-resistant hematopoietic cells, post-BMT immune reconstitution was not accelerated (Figure 2A: chimera 1). In contrast, chimeras with NK-resistant nonhematopoietic tissues and NK-susceptible hematopoietic cells displayed accelerated post-BMT immune reconstitution (Figure 2A: chimera 2). Because of the well-known key role of DCs in the activation of NK cell effector functions,15,16 we constructed recipient chimeras in which DCs were the only recipient cell type that were either resistant or susceptible to donor NK cell alloreactivity (Figure 2A: chimera 3 and chimera 4). Accelerated post-BMT immune reconstitution did not occur in chimeras that had NK-resistant DCs (Figure 2A: chimera 3). In contrast, accelerated post-BMT immune reconstitution did occur in chimeras with NK-susceptible DCs (Figure 2A: chimera 4). Thus, recipient NK-susceptible DCs were necessary and sufficient for accelerated post-BMT immune recovery to occur. To investigate whether accelerated immune reconstitution was mediated by soluble factors released as a consequence of NK/DC interactions, we cocultured alloreactive NK cells from H-2d mice with NK-susceptible DCs from H-2b mice and infused the coculture supernatant into lethally irradiated recipient mice prior to the infusion of T-cell–depleted BMT (Figure 2B). As such, experiments were designed to investigate the potential effects of alloreactive NK/DC coculture supernatants; they were intentionally performed using either allogeneic or syngeneic donor-recipient transplant pairs, with identical results. Remarkably, unlike the nonalloreactive NK/DC coculture supernatant (denoted “NKsyn + DC” in Figure 2B), the alloreactive NK/DC coculture supernatant (denoted “NKallo + DC” in Figure 2B) promoted accelerated reconstitution of donor thymocytes, B lineage cells, and DCs (Figure 2B). Thus, the interaction between alloreactive NK cells and NK-susceptible DCs resulted in the production of a soluble factor that accelerated post-BMT immune reconstitution. The factor appeared to be a newly synthesized protein as the supernatant’s ability to accelerate donor immune reconstitution was abolished by trypsin treatment and because preformed intracellular proteins obtained by lysing NK cells or DCs did not promote accelerated immune reconstitution (supplemental Figure 2A). To further demonstrate that the protein was newly synthesized and to identify the cellular source of the protein (NK cells vs DCs), DNA transcription was blocked with actinomycin D either in NK cells or in DCs before their coculture. Coculture supernatants were infused into conditioned mice before BMT. Supernatants in which DNA transcription was blocked in DCs, but not in NK cells, failed to accelerate post-BMT immune reconstitution (Figure 2B). These results demonstrated that the “immune rebuilding factor” was synthesized by DCs upon attack by alloreactive NK cells. Only the infusion of supernatants obtained by coculturing DCs with alloreactive NK cells, and not, for example, with alloreactive cytotoxic T cells, accelerated immune reconstitution (supplemental Figure 2B). Thus, it would appear that an NK cell–specific signaling was required in order to trigger DCs to activate DNA transcription and synthesis of the “immune rebuilding factor.” Moreover, because the capacity to accelerate reconstitution of the immune system was lacking in supernatant of cultures in which perforin-KO NK cells were used as effectors, it appears that the NK cell–mediated killing of allogeneic DCs was indispensable for the release of the “immune rebuilding factor” in the supernatant (supplemental Figure 2B).
Infusion of donor alloreactive NK cells accelerates post-BMT immune reconstitution. (A) H-2b recipient mice were conditioned with TBI+IL2-activated alloreactive NK cells (NKallo) from H-2d mice or with TBI+IL2-activated syngeneic (nonalloreactive) H-2b NK cells (NKsyn). Mice were then transplanted with T-cell–depleted BM from H-2d mice. (B) Recovery of BM B220+/cytoplasmic μ (Cμ)− pro-B cells (light green bars), B220+/Cμ+/surface immunoglobulins (SIg)− pre-B cells (blue bars), and B220+/SIg+ B cells (red bars). (C) Recovery of splenic B220+/SIg+ B cells. (D) Recovery of CD4+/CD8+ double-positive (red bars), CD4+ single-positive ( blue bars), and CD8+ single-positive (light green bars) thymocytes. (E) Recovery of splenic CD3+ T cells, including γ/δ T cells, regulatory T cells (Tregs), effector memory (EM) T cells, central memory (CM) T cells, and naïve T cells. (F) Splenic CD3+ T-cell proliferation in response to allogeneic H-2b splenocytes and anti-CD3 antibody stimulation at 35 days after transplant. (G) Recovery of BM CD11c+ DCs. (H) Recovery of splenic CD11cint/B220+/GR1+ plasmacytoid DCs (red bars) and CD11chigh/B220−/GR1− myeloid DCs (blue bars). (I) Recovery of thymic CD11chigh/CD8+ DCs (red bars) and CD11chigh/CD8− DCs (blue bars). Bars: mean ± standard deviation of at least 3 independent experiments; statistics were performed with a Student t test (GraphPad Prism 5). ∗P < .05, ∗∗P < .01. Cntrl, cell counts in transplant donors.
Infusion of donor alloreactive NK cells accelerates post-BMT immune reconstitution. (A) H-2b recipient mice were conditioned with TBI+IL2-activated alloreactive NK cells (NKallo) from H-2d mice or with TBI+IL2-activated syngeneic (nonalloreactive) H-2b NK cells (NKsyn). Mice were then transplanted with T-cell–depleted BM from H-2d mice. (B) Recovery of BM B220+/cytoplasmic μ (Cμ)− pro-B cells (light green bars), B220+/Cμ+/surface immunoglobulins (SIg)− pre-B cells (blue bars), and B220+/SIg+ B cells (red bars). (C) Recovery of splenic B220+/SIg+ B cells. (D) Recovery of CD4+/CD8+ double-positive (red bars), CD4+ single-positive ( blue bars), and CD8+ single-positive (light green bars) thymocytes. (E) Recovery of splenic CD3+ T cells, including γ/δ T cells, regulatory T cells (Tregs), effector memory (EM) T cells, central memory (CM) T cells, and naïve T cells. (F) Splenic CD3+ T-cell proliferation in response to allogeneic H-2b splenocytes and anti-CD3 antibody stimulation at 35 days after transplant. (G) Recovery of BM CD11c+ DCs. (H) Recovery of splenic CD11cint/B220+/GR1+ plasmacytoid DCs (red bars) and CD11chigh/B220−/GR1− myeloid DCs (blue bars). (I) Recovery of thymic CD11chigh/CD8+ DCs (red bars) and CD11chigh/CD8− DCs (blue bars). Bars: mean ± standard deviation of at least 3 independent experiments; statistics were performed with a Student t test (GraphPad Prism 5). ∗P < .05, ∗∗P < .01. Cntrl, cell counts in transplant donors.
Donor alloreactive NK cells trigger recipient DCs to synthesize proteins that accelerate post-BMT immune reconstitution. (A) Recipient murine chimeras were constructed in which hematopoietic lineage cells and nonhematopoietic tissues differed in their MHC class I, thus rendering tissues either potentially susceptible (H-2b) or resistant (H-2d or H-2d/b) to donor (H-2d) NK cell alloreactivity. Chimeras were conditioned and received NK cells and BMT from H2d mice. Illustrations show potential target cell susceptibility (→) or resistance (→|) to NK cell alloreactivity for each individual chimera. Post-BMT cell counts showed that, unlike NK-susceptible nonhematopoietic tissue (chimera 1), NK-susceptible hematopoietic cells were necessary for accelerated immune reconstitution (chimera 2). In particular, NK-susceptible DCs were the only recipient hematopoietic lineage cell that was necessary for accelerated immune rebuilding; accelerated post-BMT immune reconstitution did not occur in chimeras that had NK-resistant DCs (chimera 3), whereas accelerated post-BMT immune reconstitution did occur in chimeras with NK-susceptible DCs (chimera 4). (B) H-2d NK cells (NKallo) were cocultured with allogeneic H-2b DCs to generate alloreactive NK/DC coculture supernatants. In control experiments, in order to obtain nonalloreactive NK/DC coculture supernatants, H-2d NK cells (NKsyn) were cocultured with syngeneic H-2d DCs. The supernatants were infused into lethally irradiated mice before BMT. Infusion of alloreactive NK/DC coculture supernatants accelerated post-BMT immune recovery. Infusion of supernatants from alloreactive NK/DC cocultures in which DNA transcription was blocked in DCs by actinomycin D (actD), but not in NK cells, failed to accelerate immune reconstitution (cell counts at day +20 are shown). (C) Human alloreactive NK/DC coculture supernatants (NKallo), obtained by coculturing alloreactive NK cell clones (from HLA class I C1/C2 group heterozygous individuals) and NK-susceptible DCs (from HLA class I C group homozygous individuals), were added to thymocyte/TEC cocultures. Unlike supernatants obtained from human nonalloreactive (autologous) NK/DC combinations (NKauto), human alloreactive NK/DC coculture supernatants increased human thymocyte counts in vitro. DNA transcription blockade in DCs, but not in NK cells, prevented the increase in human thymocyte counts (cell counts at day 10 of culture are shown). ∗P < .05, ∗∗P < .01.
Donor alloreactive NK cells trigger recipient DCs to synthesize proteins that accelerate post-BMT immune reconstitution. (A) Recipient murine chimeras were constructed in which hematopoietic lineage cells and nonhematopoietic tissues differed in their MHC class I, thus rendering tissues either potentially susceptible (H-2b) or resistant (H-2d or H-2d/b) to donor (H-2d) NK cell alloreactivity. Chimeras were conditioned and received NK cells and BMT from H2d mice. Illustrations show potential target cell susceptibility (→) or resistance (→|) to NK cell alloreactivity for each individual chimera. Post-BMT cell counts showed that, unlike NK-susceptible nonhematopoietic tissue (chimera 1), NK-susceptible hematopoietic cells were necessary for accelerated immune reconstitution (chimera 2). In particular, NK-susceptible DCs were the only recipient hematopoietic lineage cell that was necessary for accelerated immune rebuilding; accelerated post-BMT immune reconstitution did not occur in chimeras that had NK-resistant DCs (chimera 3), whereas accelerated post-BMT immune reconstitution did occur in chimeras with NK-susceptible DCs (chimera 4). (B) H-2d NK cells (NKallo) were cocultured with allogeneic H-2b DCs to generate alloreactive NK/DC coculture supernatants. In control experiments, in order to obtain nonalloreactive NK/DC coculture supernatants, H-2d NK cells (NKsyn) were cocultured with syngeneic H-2d DCs. The supernatants were infused into lethally irradiated mice before BMT. Infusion of alloreactive NK/DC coculture supernatants accelerated post-BMT immune recovery. Infusion of supernatants from alloreactive NK/DC cocultures in which DNA transcription was blocked in DCs by actinomycin D (actD), but not in NK cells, failed to accelerate immune reconstitution (cell counts at day +20 are shown). (C) Human alloreactive NK/DC coculture supernatants (NKallo), obtained by coculturing alloreactive NK cell clones (from HLA class I C1/C2 group heterozygous individuals) and NK-susceptible DCs (from HLA class I C group homozygous individuals), were added to thymocyte/TEC cocultures. Unlike supernatants obtained from human nonalloreactive (autologous) NK/DC combinations (NKauto), human alloreactive NK/DC coculture supernatants increased human thymocyte counts in vitro. DNA transcription blockade in DCs, but not in NK cells, prevented the increase in human thymocyte counts (cell counts at day 10 of culture are shown). ∗P < .05, ∗∗P < .01.
To probe the existence of a human homolog of “the immune rebuilding factor,” NK cell clones were generated from individuals who possessed both HLA class I C1 and C2 group alleles (C group heterozygous individuals) and, therefore, possessed NK cells that were potentially alloreactive against targets from HLA class I C1 or C2 allele group homozygous individuals.4-6,13 NK clones that were alloreactive against DCs from individuals homozygous for either the HLA C1 or C2 allele groups were cocultured with target DCs. When added to cultures of human TECs and human thymocytes,14 supernatants from such NK/DC cocultures increased the cellularity of thymocytes (Figure 2C). As in mice, DNA transcription blockade in DCs, but not in NK cells, prevented the increase in human thymocyte counts (Figure 2C). Also, trypsin treatment of human alloreactive NK/DC coculture supernatants abolished their ability to increase thymocyte cellularity (supplemental Figure 2C). Likewise, NK cell or DC lysates were not effective at increasing thymocyte cellularity (supplemental Figure 2C). Finally, only supernatants obtained by coculturing DCs with alloreactive NK cells, and not, for example, cytotoxic T cells, were able to increase thymocyte counts (supplemental Figure 2D).
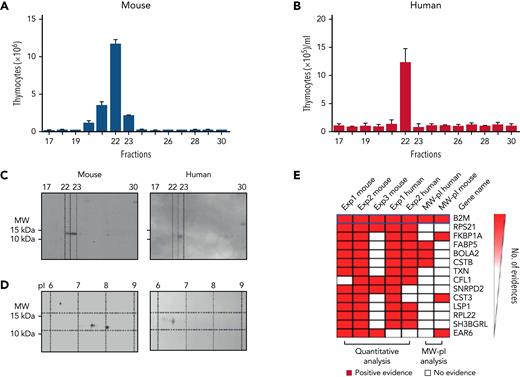
To identify newly synthesized proteins with immune rebuilding activity, we performed 2 parallel NK/DC coculture experiments: 1 in the presence and the other in the absence of 35S-methionine. Culture supernatants were fractionated by HIC and RP chromatography. One fraction (out of the 30 murine and 30 human fractions that were obtained in the nonradioactive experiment) displayed the most robust biological effect. Such fraction enhanced murine thymocyte (Figure 3A) and T-cell, B-cell, and DC counts in vivo (not shown) and increased human thymocyte cellularity in vitro in the human TEC/thymocyte cultures (Figure 3B). Then, HIC+RP chromatography fractions from the radioactive experiment were subjected to SDS-PAGE and autoradiography. An 11 to 12 kDa molecular weight (MW) protein was detected in the murine and human fractions that corresponded to the fractions that exerted maximal immune rebuilding activity in the nonradioactive experiment (Figure 3C). Two-dimensional electrophoresis showed the 11 to 12 kDa MW protein had pI of 7.5 and 8 in murine samples and 6 to 7 in human samples (Figure 3D). Subsequently, proteins were extracted from the area of interest of the nonradioactive SDS-PAGE gel, digested, and analyzed by high-resolution mass spectrometry. To identify the molecular nature of the “immune rebuilding factor” among the hundreds of proteins detected by mass spectrometry, the following ranking criteria were applied: (1) ≥1.5-fold increase in protein content in supernatants from alloreactive NK/DC cocultures in comparison with supernatants from nonalloreactive NK/DC cocultures, (2) 11 to 12 kDa MW, (3) pI of 6 to 7 for the human and 7.5 and 8 for the murine protein, and (4) detection of the protein displaying the above biochemical features in all murine (n = 3) and human (n = 2) experiments. Among the 30 top-ranking proteins (supplemental Table 1) out of the 853 that were detected by mass-spectrometry, we preliminarily tested FKBP1A because it is the target of tacrolimus and has a plethora of immune-modulatory functions,17 in spite of the fact that it did not fulfill all the ranking criteria because it had pI that differed from those detected in our experiments. We immune-depleted alloreactive NK/DC coculture supernatant with anti-FKBP1A antibodies. The immune rebuilding effect of the supernatant was not affected (not shown).
Biochemical analyses demonstrate that the murine and human “immune rebuilding factor” is B2M. (A-B) Murine and human NK/DC coculture supernatants were fractionated by HIC+RP. One out of the 30 fractions (fraction 22) displayed the most robust biological effect with enhanced murine thymocyte, T-cell, B-cell, and DC counts in vivo and increased human thymocyte cellularity in vitro in the human TEC/thymocyte cultures (the in vivo increase in murine T cells, B cells, and DCs is not shown). (C-D) Two parallel NK/DC coculture experiments were performed, 1 with and 2 without 35S-methionine, and supernatants were fractionated by HIC+RP chromatography. (C) Fractions from the radioactive experiment were subjected to SDS-PAGE and autoradiography. An 11 to 12 kDa MW protein was detected in the fractions that corresponded to those that exerted maximal immune rebuilding activity in the nonradioactive experiment. (D) Two-dimensional electrophoresis showed the 11 to 12 kDa MW protein had isoelectric points (pI) of 7.5 and 8 for murine and 6 to 7 for human samples. (E) The 14 top-ranking proteins identified by mass spectrometry that displayed a ≥1.5-fold increase in protein content in alloreactive NK/DC coculture supernatants as compared with nonalloreactive NK/DC coculture supernatants. Proteins were ranked according to presence of (1) 11 to 12 kDa MW, (2) pI of 7.5 and 8 for murine and 6 to 7 for human samples (on top of panel E, the combination of the above biochemical features is denoted as “MW-pI”), and (3) detection of the protein displaying the above biochemical features in all murine and human experiments. The only protein that possessed all of these features was B2M.
Biochemical analyses demonstrate that the murine and human “immune rebuilding factor” is B2M. (A-B) Murine and human NK/DC coculture supernatants were fractionated by HIC+RP. One out of the 30 fractions (fraction 22) displayed the most robust biological effect with enhanced murine thymocyte, T-cell, B-cell, and DC counts in vivo and increased human thymocyte cellularity in vitro in the human TEC/thymocyte cultures (the in vivo increase in murine T cells, B cells, and DCs is not shown). (C-D) Two parallel NK/DC coculture experiments were performed, 1 with and 2 without 35S-methionine, and supernatants were fractionated by HIC+RP chromatography. (C) Fractions from the radioactive experiment were subjected to SDS-PAGE and autoradiography. An 11 to 12 kDa MW protein was detected in the fractions that corresponded to those that exerted maximal immune rebuilding activity in the nonradioactive experiment. (D) Two-dimensional electrophoresis showed the 11 to 12 kDa MW protein had isoelectric points (pI) of 7.5 and 8 for murine and 6 to 7 for human samples. (E) The 14 top-ranking proteins identified by mass spectrometry that displayed a ≥1.5-fold increase in protein content in alloreactive NK/DC coculture supernatants as compared with nonalloreactive NK/DC coculture supernatants. Proteins were ranked according to presence of (1) 11 to 12 kDa MW, (2) pI of 7.5 and 8 for murine and 6 to 7 for human samples (on top of panel E, the combination of the above biochemical features is denoted as “MW-pI”), and (3) detection of the protein displaying the above biochemical features in all murine and human experiments. The only protein that possessed all of these features was B2M.
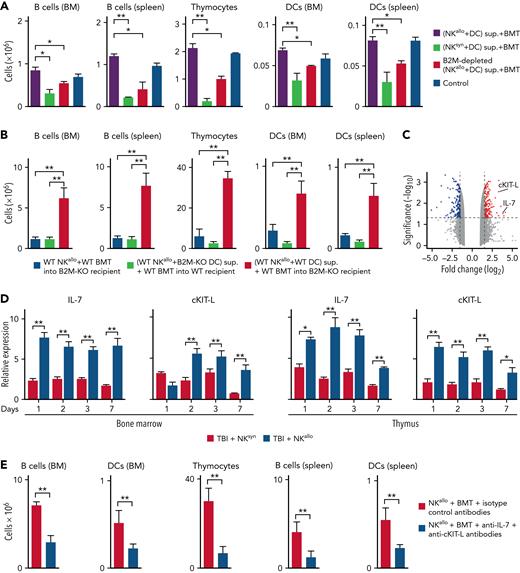
Only 1 protein, B2M, fulfilled all the ranking criteria18,19 (Figure 3E; supplemental Table 1). We next sought functional evidence for B2M’s role in accelerating immune reconstitution. In the mouse, anti-B2M antibody depletion reduced the immune rebuilding ability of alloreactive NK/DC coculture supernatants, thus indicating B2M played a role in posttransplant immune reconstitution (Figure 4A). Most importantly, genetic evidence was obtained in B2M-KO H-2b mice (Figure 4B). B2M-KO H-2b mice were lethally irradiated and were given alloreactive NK cells and a T-cell–depleted BMT from WT H-2d mice. In this model, accelerated immune reconstitution was not observed. Accelerated post-BMT immune rebuilding was also not observed when lethally irradiated WT H-2b mice were given an infusion of supernatants from cocultures of alloreactive WT H-2d NK cells and B2M-KO H-2b DCs. In contrast, infusion of supernatants obtained from cocultures of alloreactive WT H-2d NK cells and WT H-2b DCs endowed B2M-KO mice with the capacity to undergo accelerated post-BMT immune reconstitution (Figure 4B).
B2M triggers accelerated post-BMT immune reconstitution. (A) Anti-B2M antibody depletion reduced the immune rebuilding ability of alloreactive NK/DC coculture supernatants. (B) When B2M-KO H-2b mice were lethally irradiated and given alloreactive NK cells and a T-cell–depleted BMT from WT H-2d mice, no accelerated post-BMT immune reconstitution occurred. Also, no accelerated post-BMT immune reconstitution occurred when supernatants from cocultures of alloreactive WT H-2d NK cells and B2M-KO DCs were infused in WT recipient mice. In contrast, infusion of supernatants from cocultures of alloreactive WT H-2d NK cells and WT H-2b DCs restored the B2M-KO mouse ability to undergo accelerated post-BMT immune reconstitution. (C) RNA sequencing (RNA-seq) volcano plot analysis displayed genes that were differentially expressed in the BM of mice 7 days after they had received TBI and infusion of alloreactive NK/DC coculture supernatants (without BMT rescue) vs infusion of nonalloreactive NK/DC coculture supernatants. RNA-seq analysis revealed significant upregulation of 2 master regulators of lymphocyte development, namely IL-7 and cKIT-L. (D) qPCR showed IL-7 and cKIT-L were stably upregulated (for 1 week) in the BM and thymus of lethally irradiated, alloreactive NK cell–treated mice. (E) Infusion of anti–IL-7 plus anti–cKIT-L antibodies into BMT-transplanted mice prevented accelerated immune reconstitution. ∗P < .05, ∗∗P < .01.
B2M triggers accelerated post-BMT immune reconstitution. (A) Anti-B2M antibody depletion reduced the immune rebuilding ability of alloreactive NK/DC coculture supernatants. (B) When B2M-KO H-2b mice were lethally irradiated and given alloreactive NK cells and a T-cell–depleted BMT from WT H-2d mice, no accelerated post-BMT immune reconstitution occurred. Also, no accelerated post-BMT immune reconstitution occurred when supernatants from cocultures of alloreactive WT H-2d NK cells and B2M-KO DCs were infused in WT recipient mice. In contrast, infusion of supernatants from cocultures of alloreactive WT H-2d NK cells and WT H-2b DCs restored the B2M-KO mouse ability to undergo accelerated post-BMT immune reconstitution. (C) RNA sequencing (RNA-seq) volcano plot analysis displayed genes that were differentially expressed in the BM of mice 7 days after they had received TBI and infusion of alloreactive NK/DC coculture supernatants (without BMT rescue) vs infusion of nonalloreactive NK/DC coculture supernatants. RNA-seq analysis revealed significant upregulation of 2 master regulators of lymphocyte development, namely IL-7 and cKIT-L. (D) qPCR showed IL-7 and cKIT-L were stably upregulated (for 1 week) in the BM and thymus of lethally irradiated, alloreactive NK cell–treated mice. (E) Infusion of anti–IL-7 plus anti–cKIT-L antibodies into BMT-transplanted mice prevented accelerated immune reconstitution. ∗P < .05, ∗∗P < .01.
Given that multiple cells of the hematopoietic lineage, such as B lineage cells, developing T cells, and DCs, were involved in the immune rebuilding effect, we hypothesized that the BM environment played a primary role in mediating the accelerated immune recovery. Therefore, to identify the molecular mechanisms underlying the regenerative effects induced by B2M, we performed RNA-seq analyses (shown as a volcano plot in Figure 4C) followed by gene set enrichment analysis on BM from mice that had been lethally irradiated but not rescued by BMT and had received an infusion of alloreactive NK/DC coculture supernatants vs infusion of nonalloreactive (syngeneic) NK/DC coculture supernatants. The list of differentially regulated genes is shown in the BM gene expression profiling table (BM gene expression profiling, supplemental Data 1). Among the 20 most upregulated genes, IL-7 and cKIT-L stood out because they are well-known master regulators of lymphocyte development.20-22 Indeed, IL-7 and cKIT-L messenger RNA quantification by qPCR confirmed that these cytokines were stably upregulated (for 1 week) in the BM and thymus of lethally irradiated, alloreactive NK cell–treated mice (Figure 4D). IL-7 and cKIT-L were indispensable for faster post-BMT immune reconstitution as combined infusion of blocking anti–IL-7 and anti–cKIT-L antibodies hindered accelerated immune reconstitution of BMT-transplanted mice (Figure 4E). Interestingly, gene set enrichment analysis revealed that several biological processes, including cell cycle phase transition, response to cytokine, biosynthetic metabolic processes, and leukocyte immunity, were induced after infusion of alloreactive NK/DC coculture supernatants (supplemental Figure 3 and GEAS table results, supplemental Data 2).
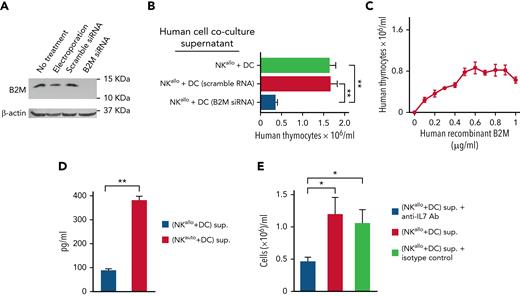
Comparable results were obtained using human alloreactive NK cells cocultured with human DCs in which B2M gene expression was silenced by short interfering-RNA (Figure 5A). Supernatants from cocultures of alloreactive NK cells and B2M-silenced DCs failed to increase thymocyte cellularity in the human TEC/thymocyte culture system (Figure 5B). Further evidence for the involvement of B2M in the accelerated immune rebuilding was obtained by the use of recombinant B2M. Unfortunately, the use of murine recombinant B2M did not provide informative results (data not shown), probably because commercially available murine recombinant B2M molecules are histidine-tagged, a feature that is known to result in diminished or altered biological activity.23,24 In contrast, a commercially available human recombinant B2M, which is not histidine-tagged and is expressed in Escherichia coli as the 14.0 kDa B2M precursor,25 did exert a dose-dependent effect on the increase in thymocyte cellularity in the TEC/thymocyte culture system. However, the effect mediated by 14.0 kDa B2M precursor was smaller (one-third) than that obtained by using alloreactive NK/DC coculture supernatants (Figure 5C).
Genetic and functional evidence that human B2M triggers IL-7 production by human TECs and augments thymocyte counts. (A) Western blot of B2M developed by Enhanced ChemiLuminescence shows the absence of the B2M protein in DCs in which the human B2M gene was silenced with siRNA vs its presence in control DCs (untreated or electroporated or treated with scramble siRNA). (B) Supernatants from cocultures of human alloreactive NK cells and B2M-silenced DCs failed to increase human thymocyte cellularity (see the lane labeled “B2M siRNA”). (C) Increase in human thymocyte cellularity upon addition of human recombinant B2M in the TEC/thymocyte culture system. (D) Human alloreactive or nonalloreactive NK cell clones were cocultured with human DCs. NK/DC coculture supernatants were added to human TECs. Unlike supernatants from nonalloreactive (auto) NK/DC cocultures, the supernatants from alloreactive NK/DC (allo) cocultures promoted IL-7 production by TECs. (E) When thymocytes were cultured with TECs, the addition of an anti–IL-7 Ab prevented the increase in thymocyte counts mediated by an alloreactive NK/DC coculture supernatant. Bars: mean ± standard deviation of at least 3 independent experiments; statistics were performed with a Student test (GraphPad Prism 5). ∗P < .05; ∗∗P < .01. siRNA, short interfering-RNA.
Genetic and functional evidence that human B2M triggers IL-7 production by human TECs and augments thymocyte counts. (A) Western blot of B2M developed by Enhanced ChemiLuminescence shows the absence of the B2M protein in DCs in which the human B2M gene was silenced with siRNA vs its presence in control DCs (untreated or electroporated or treated with scramble siRNA). (B) Supernatants from cocultures of human alloreactive NK cells and B2M-silenced DCs failed to increase human thymocyte cellularity (see the lane labeled “B2M siRNA”). (C) Increase in human thymocyte cellularity upon addition of human recombinant B2M in the TEC/thymocyte culture system. (D) Human alloreactive or nonalloreactive NK cell clones were cocultured with human DCs. NK/DC coculture supernatants were added to human TECs. Unlike supernatants from nonalloreactive (auto) NK/DC cocultures, the supernatants from alloreactive NK/DC (allo) cocultures promoted IL-7 production by TECs. (E) When thymocytes were cultured with TECs, the addition of an anti–IL-7 Ab prevented the increase in thymocyte counts mediated by an alloreactive NK/DC coculture supernatant. Bars: mean ± standard deviation of at least 3 independent experiments; statistics were performed with a Student test (GraphPad Prism 5). ∗P < .05; ∗∗P < .01. siRNA, short interfering-RNA.
In the human TEC/thymocyte culture system, alloreactive NK/DC coculture supernatants promoted IL-7 production by TECs (Figure 5D), and the addition of an anti–IL-7 antibody prevented the increase in thymocyte cellularity, thus indicating that thymocyte expansion promoted by alloreactive NK/DC coculture supernatants was IL-7 dependent in this in vitro system (Figure 5E).
Discussion
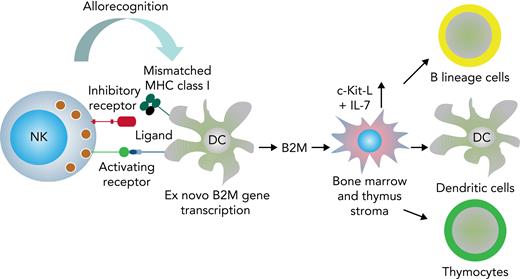
The present study uncovers a novel, NK cell–based immunotherapeutic intervention to obviate post–bone marrow transplant immune deficiency. In murine allogeneic bone marrow transplantation models, the infusion of ex vivo–expanded donor alloreactive NK cells triggered B2M production by recipient DCs that in turn elicited the release of IL-7 and cKIT-L from thymic and BM stroma. IL-7 and cKIT-L greatly accelerated reconstitution of donor-type, developing, and mature T and B cells, as well as of DCs in the thymus, BM, and spleen (see Figure 6 for a graphic summary of data). Comparable results were obtained in a human in vitro thymocyte/TEC culture system; that is, a human alloreactive NK/DC coculture supernatant promoted IL-7 production by TECs, which in turn supported thymocyte cellularity.
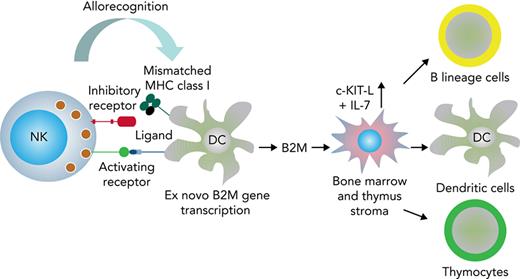
Graphic summary of data. During the interaction between NK cells and MHC-mismatched DCs, the mismatched MHC may not be recognized by NK cell inhibitory receptors, and the lytic action elicited by activating receptors is allowed to proceed.4,5 The NK cell alloreaction triggers DCs to transcribe the B2M gene and to synthesize and release B2M. B2M signals thymic and BM stroma to produce cKIT-L and IL-7 that mediate accelerated post-BMT immune reconstitution.
Graphic summary of data. During the interaction between NK cells and MHC-mismatched DCs, the mismatched MHC may not be recognized by NK cell inhibitory receptors, and the lytic action elicited by activating receptors is allowed to proceed.4,5 The NK cell alloreaction triggers DCs to transcribe the B2M gene and to synthesize and release B2M. B2M signals thymic and BM stroma to produce cKIT-L and IL-7 that mediate accelerated post-BMT immune reconstitution.
Evidence for the unprecedented involvement of B2M in immune reconstitution was obtained by the observation that (1) chromatography fractionation of alloreactive NK/DC coculture supernatants identified a protein with MW and pI of B2M. (2) A high-sensitivity mass spectrometry analysis identified a protein with the amino acid sequences specific of B2M. (3) Anti-B2M antibody depletion of NK/DC coculture supernatants abrogated the immune rebuilding effect. (4) The B2M gene is indispensable for accelerated immune reconstitution. B2M KO mice were unable to undergo accelerated immune reconstitution, and the infusion of WT NK/DC coculture supernatants reversed their inability to undergo accelerated immune reconstitution. Similarly, silencing the human B2M gene in human DCs before coculture with alloreactive NK cells prevented the increase in thymocyte cellularity in the human in vitro system. (5) The human recombinant 14.0 kDa B2M precursor25 increased thymocyte cellularity in an in vitro TEC/thymocyte culture system. The partial immune rebuilding effect exerted by the human recombinant B2M, compared with the B2M synthesized by DCs, might be due to the fact that the recombinant B2M had a molecular weight of 14.0 kDa because it included the whole precursor sequence with the signal peptide for secretion. Moreover, in the E. coli expression system, no posttranslational modifications are usually inserted in the expressed proteins due to the diversity of the molecular machinery for posttranslational modifications, including proteolytic cleavage. According to our analysis, the protein candidate responsible of the immune reconstitution and identified as B2M had a molecular weight of ∼12.0 kDa, corresponding to the mature sequence of B2M as confirmed by SDS-PAGE and mass spectrometry.25 It is, therefore, possible that the actual effector is the mature protein and not the precursor. Furthermore, 2-dimensional electrophoresis experiments showed that B2M is present as 2 spots with different pI, a possible indication of DC-specific posttranslational modifications that may happen before secretion and may be involved in the immune reconstitution effect.
B2M, recently shown to play a role in inflammation,26,27 is primarily known as a key component of MHC class I family molecules; it is necessary for their correct folding and cell surface expression enabling the formation of a stable peptide binding groove and, consequently, for recognition by CD8+ T cells and CD8+ T-cell clonal expansion. Interestingly, B2M deficiency in humans supports the idea that B2M is involved in the maturation of various components of the immune system because B2M-deficient patients exhibit broad immune incompetence involving not only CD8+ T cells but also CD4+ and B cells.28 Thus, it is conceivable that the NK/DC “crosstalk”16 and the consequent NK cell activation and killing of autologous DCs15 may lead to B2M production even under physiological conditions (an effect that our control experiments using autologous NK/DC combinations might not have been sensitive enough to detect).
To improve immune reconstitution after allogeneic hematopoietic cell transplantation, a variety of approaches have been proposed, such as adoptive T-cell therapy with nonalloreactive and/or pathogen-specific T cells, transfer of lymphoid progenitor cells, thymic grafts, enhancement of thymopoiesis by sex-steroid blockade, administration of IL-7, keratinocyte growth factor, fms-like tyrosine kinase 3 ligand, IL-22, or growth hormone.29-38 Approaches thus far tested clinically have significant limitations. Here, for the first time, we demonstrate that infusion of large doses of alloreactive NK cells promotes striking acceleration of post–bone marrow transplant immune reconstitution. As it might have been expected, we did not find any difference in immune reconstitution of patients transplanted from NK alloreactive donors and patients transplanted from non-NK alloreactive donors. In fact, it should be noted that there are very remarkable differences between our clinical transplantation protocol3,4 and the murine experiments. Human transplants were performed using purified CD34+ hematopoietic progenitor cells, which give rise to the reconstitution of NK cells that initially contain alloreactive cells in low frequencies. In contrast, the murine experiments were performed with the infusion of very large numbers of ex vivo–expanded, IL2-activated donor NK cells containing high frequencies (40%-50%) of alloreactive cells.
In conclusion, our studies uncover a novel therapeutic principle for the treatment of post–bone marrow transplant immune deficiency and suggest that future protocols that will include the adoptive transfer of large numbers of cytokine-activated, ex vivo–expanded donor alloreactive NK cells may improve the survival rate of patients undergoing haploidentical hematopoietic transplantation.
Acknowledgments
The authors thank Temistocle Ragni (Ospedale Santa Maria della Misericordia, Perugia) and Adriano Carotti (Ospedale Bambino Gesù, Roma) for thymic tissue samples obtained during corrective cardiovascular surgery. The authors also thank Massimo F. Martelli, Professor Emeritus, University of Perugia, for critical review of the manuscript and helpful advice.
This work was supported by grants from the Italian Association for Cancer Research (AIRC), the Swiss National Science Foundation, the Leukemia and Lymphoma Society, and the Italian Ministry for Further Education. Vrije Universiteit Medical Center-Cancer Center Amsterdam is acknowledged for support of the mass spectrometry infrastructure. E.V. was supported by grants from the Amy Strelzer Manasevit Research Program and AIRC.
Authorship
Contribution: L.R. designed and performed experiments and wrote paper; E.U., S.C., and R. S. performed murine transplant experiments and human cell cultures; F.S. and P.L.O. performed biochemical analyses; E.B. performed murine immune reconstitution analyses; D.C. designed and performed proteomics analyses and wrote paper; S.R.P. performed proteomics analyses; S.B. performed human RNA silencing experiments; D.R. contributed to human thymocyte culture experiments; S.P. as heart surgeon at the Ospedale Santa Maria della Misericordia, Perugia, provided human thymic tissue samples obtained during corrective cardiovascular surgery; L.B. conceived, designed, and supervised biochemical analyses and critically revised the manuscript; C.R.J. supervised proteomics analyses and critically revised the manuscript; G.A.H. designed experiments, provided constructive suggestions, and wrote paper; E.V. and A.C performed GSEA and revised the manuscript; A.P. and F.L. revised the manuscript; and A.V. conceived and supervised the project, designed experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Loredana Ruggeri, Ospedale Santa Maria della Misericordia di Perugia, Piazzale Menghini 1, 06132 Perugia, Italy; e-mail: loredana.ruggeri@ospedale.perugia.it; and Andrea Velardi, Division of Hematology and Clinical Immunology and Bone Marrow Transplantation Program, Department of Medicine and Surgery, University of Perugia, Piazza Gambuli 1, 06132 Perugia, Italy; e-mail: andrea.velardi@unipg.it.
References
Author notes
Send data sharing requests via e-mail to the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal