Key Points
A novel alloantigen-specific model has been developed and used to examine the efficacy of a prophylactic treatment regimen for FNAIT.
The resulting preclinical data provide guidance for the use of HPA-1a–specific antibodies to prevent FNAIT in humans.
Abstract
Fetal/neonatal alloimmune thrombocytopenia (FNAIT) is a life-threatening bleeding disorder caused by maternal alloantibodies directed against paternally inherited human platelet alloantigens (HPAs) present on the surface of fetal and neonatal platelets. There are currently no approved therapies for the prevention of FNAIT. We report herein the ability of 2 human HPA-1a–specific therapeutic candidates, one a polyclonal, and the other a monoclonal antibody, to prevent alloimmunization in a novel preclinical mouse model of FNAIT. Both antibody preparations effected the rapid and complete elimination of HPA-1a+ platelets from circulation and prevented the development of HPA-1a alloantibodies. HPA-1a− female mice treated prophylactically with anti–HPA-1a antibody prior to exposure to HPA-1a+ platelets gave birth to HPA-1a+/− pups with significantly improved platelet counts and no bleeding symptoms. These preclinical data establish both the potential and threshold exposure targets for prophylactic treatment with HPA-1a–specific antibodies for the prevention of FNAIT in humans.
Introduction
Fetal/neonatal alloimmune thrombocytopenia (FNAIT) is a rare, life-threatening bleeding disorder that arises when maternal antibodies specific for paternally inherited fetal platelet alloantigens cross the placenta and lead to profound depletion of fetal/neonatal platelets. FNAIT complicates ∼1 in 350 pregnancies and has been reported to lead to severe thrombocytopenia in ∼1 in 1000 to 2500 live births.1,2 Although cases of mild thrombocytopenia often resolve without incident, fetuses may unpredictably experience intracranial hemorrhage, which can cause irreversible brain damage and death. The human platelet antigen 1a (HPA-1a) is the most frequently implicated HPA for causing FNAIT, accounting for 75% to 80% of severe FNAIT cases.3-5
In some respects, FNAIT has a similar pathophysiology to that of hemolytic disease of the fetus and newborn (HDFN).6,7 In the case of HDFN, a Rhesus D (RhD)-negative pregnant patient may develop alloantibodies to paternally inherited RhD antigens expressed on fetal and neonatal red blood cells (RBCs). The maternal immunoglobulin G (IgG) alloantibodies can traverse the placenta and destroy the fetal RBCs, leading to anemia and potentially hydrops fetalis and/or death of the fetus. HDFN and FNAIT, however, diverge both in the source of fetal antigen (RhD vs HPA, respectively) and the timing of maternal alloimmunization. Although nearly all HDFN occurs in subsequent pregnancies after alloimmunization from fetal-maternal hemorrhage during a previous pregnancy, an estimated 25% or more of FNAIT cases occur without warning during gestation of the first pregnancy8-10 due to alloimmunization to human platelet antigens (HPA) as early as gestational week 17.11,12
Though treatment strategies for pregnancies following a first case of FNAIT have continued to evolve in recent decades,8,9,13,14 there are currently no approved therapies for the prevention of FNAIT. It has been hypothesized that low doses of anti–HPA-1a IgG could be used to prevent alloimmunization and subsequent FNAIT in patients at risk,7,15 much in the same way that over the last half century, low doses of antibodies to RhD have been given to at-risk patients during pregnancy and at parturition to prevent >99% of cases of hemolytic disease of the newborn (HDN).16
We recently described the development of transgenic mice expressing the human HPA-1a alloantigenic epitope on a murine GPIIIa backbone.17 Immunization of wild-type (WT) female mice with platelets from HPA-1a transgenic mice induces the generation of high-titer anti–HPA-1a alloantibodies that can cross the placenta and recapitulate many of the relevant clinical features of FNAIT.18 The purpose of the present investigation was to use this alloantigen-specific preclinical animal model to evaluate the ability of HPA-1a–specific human antibodies to prevent maternal alloimmunization and the development of FNAIT. The preclinical efficacy studies presented herein establish the potential for prophylactic administration of low-dose anti–HPA-1a antibody therapy to prevent FNAIT in at-risk patients during pregnancy and parturition.
Materials and methods
Antibodies
RLYB211 (EudraCT Number: 2019-003459-12) is a hyperimmune polyclonal IgG preparation containing HPA-1a–specific antibodies derived from plasma obtained from patients who had become alloimmunized as a result of a prior pregnancy.15 RLYB212 (EudraCT Number 2021-003196-34) is a novel recombinant human monoclonal antibody directed against the HPA-1a epitope developed from an antibody sequence cloned from the B cell of a patient who had become HPA-1a alloimmunized during pregnancy.19
To enable the direct comparison of the efficacy of RLYB211 and RLYB212, the anti–HPA-1a binding capacity of each antibody preparation used in these experiments was measured in a qualified enzyme-linked immunosorbent assay using a purified, recombinant chimeric monomer of integrin β3 (cβ3) as the HPA-1a capture reagent.20,21 Binding was normalized against the World Health Organization standard (NIBSC code: 03/152) to establish the binding potency of each antibody in IU.
Mice
WT BALB/c female mice (8-12 weeks of age) were purchased from Charles River laboratories. C57BL/6 mice expressing murine GPIIIa harboring T30→A, S32→P, Q33→L, N39→D, and M470→Q (hereafter referred to as APLDQ GPIIIa) mouse-to-human amino acid substitutions within the plexin semaphorin integrin and adjacent epidermal growth factor 1 domains were generated and maintained as previously described.17 All mice were housed in the Biological Resource Center at the Medical College of Wisconsin, a facility fully approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. All protocols were carried out in compliance with the human and animal experimentation guidelines of the US Department of Health and Human Services and in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Platelet and plasma preparation
Normal human IgG was prepared from blood samples from healthy volunteers as approved by the institutional review board of the Medical College of Wisconsin after obtaining informed consent in accordance with the Declaration of Helsinki. Mouse blood was drawn from the inferior vena cava of anesthetized mice into a syringe containing 3.8% sodium citrate, diluted 1:1 with modified Tyrode–N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (Tyrode-HEPES) buffer (10 mM HEPES [pH 7.4], 12 mM NaHCO3, 137 mM NaCl, 2.7 mM KCl, 5 mM glucose, 0.25% bovine serum albumin), layered onto Fico/Lite for Platelets (Atlanta Biologicals, Flowery Branch, GA), and centrifuged for 15 minutes at 700g. Platelets were collected from the interface, washed once in Tyrode-HEPES buffer containing 50 ng/mL prostaglandin E1 and 1 mM EDTA, pH 7.4, and resuspended in phosphate-buffered saline (PBS). Whole human and mouse blood were diluted (1:3) with PBS and centrifuged at 700g for 8 minutes, after which plasmas were collected and stored at −20°C until use.
Flow cytometric analysis
Binding of RLYB211 and RLYB212 to HPA-1a/a and HPA-1b/b human platelets, and WT and APLDQ mouse platelets, was evaluated by flow cytometry. Antibody binding was detected using fluorescein isothiocyanate (FITC)-labeled goat F(ab′)2 anti-human IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) using an Accuri C6 flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Platelet labeling and in vivo survival studies
Platelets were isolated from APLDQ C56BL/6 mice and fluorescently labeled with 5 μM 5-chloromethyl fluorescein diacetate (CMFDA) (Thermo Fisher Scientific, Waltham, MA) for 45 minutes in the dark following previously described methods.22 1 × 108 CMFDA-labeled platelets were transfused into WT BALB/c mice, and blood samples were collected 30 minutes after platelet transfusion to define the baseline of circulating CMFDA-labeled APLDQ platelets, which was assigned a value of 100%. One hour after platelet introduction, varying doses of RLYB211, RLYB212, or nonbinding human IgG were introduced by tail vein injection. Blood samples were collected from the submandibular vein using EDTA-coated Sarstedt Microvette capillary blood collection tubes post–platelet transfusion and pre–antibody injection, 5 hours post–antibody injection, and 24 hours post–antibody injection. Survival of CMFDA-labeled APLDQ platelets in the mouse circulation was determined by flow cytometry.
Prophylactic regimens
Female WT BALB/c mice (age, 8-12 weeks) were immunized by tail vein transfusion with 1 × 108 APLDQ platelets in PBS, with or without prior tail vein injection of prophylactic RLYB211, RLYB212, or nonbinding human IgG. Blood samples were collected before (prebleed) and after the platelet transfusion on days 7, 14, and 21. Plasma from these samples was examined for levels of mouse anti-APLDQ using FITC-conjugated goat (Fab′)2 anti-mouse IgG (Jackson ImmunoResearch Laboratories). The presence of residual RLYB211 and 212 in circulation was assessed using FITC-labeled goat F(ab′)2 anti-human IgG (Jackson ImmunoResearch Laboratories).
To assess the ability of anti–HPA-1a treatment to sustain prophylactic benefit in the event of repeated antigen exposures, a second prophylaxis treatment and platelet challenge was administered in the RLYB211 treatment group on day 21. After the last blood samples were collected, female BALB/c mice from the RLYB211 prophylaxis treatment groups were bred with APLDQ C57BL/6 males. Pups were monitored for signs of hemorrhage for 48 hours after birth, during which time blood was collected from the dam and submandibular vein of the pups into EDTA-coated capillary tubes or heparinized microhematocrit capillary tubes (Thermo Fisher Scientific). Platelet counts were determined using a scil Vet abc animal blood counter (scil Animal Care Company, Gurnee, IL), and plasma was harvested by centrifugation and subjected to APLDQ-specific antibody analysis.
Statistical analyses
The Mann-Whiney U test was used to compare different groups used for analysis of platelet counts and antibody levels using the statistical packages SAS 9.4 and SPSS 28. Unadjusted double-sided P values are shown, with P < .05 considered statistically significant.
Results
Relative abilities of polyclonal and monoclonal HPA-1a–specific antibodies to saturate HPA-1a epitopes on human and APLDQ transgenic mouse platelets
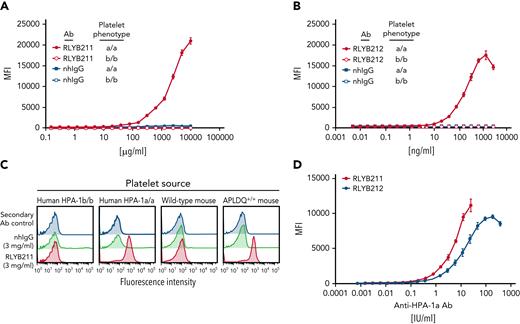
We previously reported the generation of transgenic mice that were humanized to express the HPA-1a epitope on a murine GPIIIa backbone (hereafter termed APLDQ GPIIIa) and used them to demonstrate that the human HPA-1a–specific monoclonal antibody (mAb) 26.4 binds to APLDQ, but not WT, murine platelets.17 RLYB212 was developed from mAb 26.4 and retains the parental binding characteristics. RLYB211 is a hyperimmune anti–HPA-1a polyclonal IgG preparation15; however, its ability to bind the APLDQ epitope has not been previously characterized. As shown in Figure 1, both antibodies bind specifically to HPA-1a/a, but not HPA-1b/b, human platelets, as expected; and also selectively bind to APLDQ (HPA-1a+), but not WT, murine platelets.
Binding isotherms of RLYB211 and RLYB212 to human and mouse platelets expressing HPA-1 alloantigens. (A-B) Washed human platelets from HPA-1a/a and HPA-1b/b individuals were incubated with serially diluted RLYB211, RLYB212, or normal human IgG (nhIgG) and incubated for 1 hour at room temperature. Bound antibodies were detected using a 1/200 dilution of FITC-conjugated goat anti-human IgG. Note the specificity of RLYB211 and RLYB212 for the HPA-1a alloantigen. Note also that RLYB211 is not able to saturate all available GPIIb-IIIa receptors, even at 10 mg/mL, because the HPA-1a–specific antibody is only a small fraction of the total polyclonal IgG present in RLYB211. (C) Platelets from the indicated species and having the indicated phenotypes were incubated with normal human IgG or RLYB211. Note that in addition to the specificity of RLYB211 for HPA-1a expressed on GPIIIa on the surface of human platelets, RLYB211 is also specific for the APLDQ form of mouse GPIIIa on the surface of humanized, but not WT, mouse platelets. (D) APLDQ platelets were incubated with increasing concentrations of polyclonal RLYB211 or the monospecific monoclonal antibody RLYB212, and antibody binding was quantified using flow cytometry to generate saturation-binding curves. Note that the addition of ∼4 IU/mL of either antibody is well below saturation and results in occupancy of <10% of the available GPIIb-IIIa receptors.
Binding isotherms of RLYB211 and RLYB212 to human and mouse platelets expressing HPA-1 alloantigens. (A-B) Washed human platelets from HPA-1a/a and HPA-1b/b individuals were incubated with serially diluted RLYB211, RLYB212, or normal human IgG (nhIgG) and incubated for 1 hour at room temperature. Bound antibodies were detected using a 1/200 dilution of FITC-conjugated goat anti-human IgG. Note the specificity of RLYB211 and RLYB212 for the HPA-1a alloantigen. Note also that RLYB211 is not able to saturate all available GPIIb-IIIa receptors, even at 10 mg/mL, because the HPA-1a–specific antibody is only a small fraction of the total polyclonal IgG present in RLYB211. (C) Platelets from the indicated species and having the indicated phenotypes were incubated with normal human IgG or RLYB211. Note that in addition to the specificity of RLYB211 for HPA-1a expressed on GPIIIa on the surface of human platelets, RLYB211 is also specific for the APLDQ form of mouse GPIIIa on the surface of humanized, but not WT, mouse platelets. (D) APLDQ platelets were incubated with increasing concentrations of polyclonal RLYB211 or the monospecific monoclonal antibody RLYB212, and antibody binding was quantified using flow cytometry to generate saturation-binding curves. Note that the addition of ∼4 IU/mL of either antibody is well below saturation and results in occupancy of <10% of the available GPIIb-IIIa receptors.
To assess the efficacy of RLYB211 and RLYB212, the potency of each antibody preparation was determined in a qualified enzyme-linked immunosorbent assay for binding to HPA-1a. Binding potency was normalized against the World Health Organization anti–HPA-1a standard and is represented as IU. Concentration-binding isotherms were assessed for RLYB211 and RLYB212 (in IU/mL) against APLDQ homozygous platelets. The dynamic range of the binding-response curve was comparable (within twofold) for each of these 2 antibodies (Figure 1D). RLYB212 was able to fully saturate GPIIb-IIIa receptors at concentrations greater than ∼100 IU/mL (Figure 1D); however, receptor saturation with RLYB211 could not be established due to the low percentage of HPA-1a–specific antibodies in the polyclonal antibody (relative to total IgG). Note that the effective doses used in subsequent in vivo experiments are projected to occupy less than one-third of exposed GPIIb-IIIa receptors on transfused platelets. As such, the antibodies would be predicted to have only a small effect on the ability of platelets to aggregate, abolition of which requires >80% receptor occupancy.23-25
RLYB211 and RLYB212 eliminate circulating HPA-1a+ murine platelets, prevent alloimmunization, and rescue neonatal platelet counts
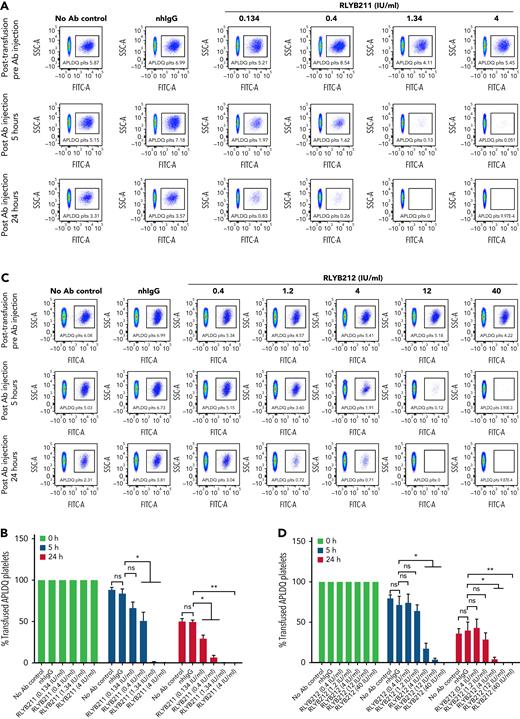
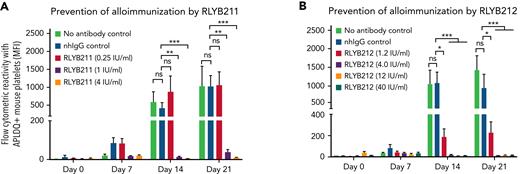
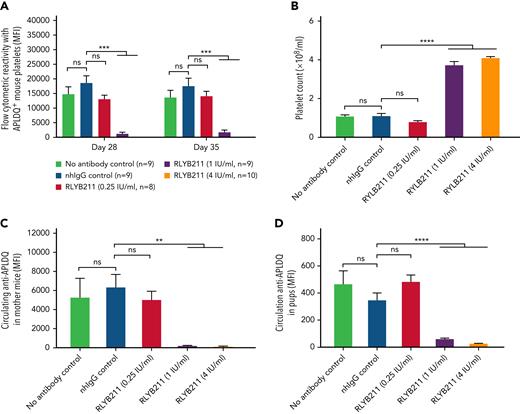
To evaluate the relative ability of either polyclonal or monoclonal HPA-1a–specific antibodies to remove foreign HPA-1a antigen from circulation, each antibody was administered by IV injection into WT BALB/c mice 1 hour after IV transfusion of 1 × 108 CMFDA-labeled APLDQ-homozygous platelets. In 5 repeated experiments, doses estimated to yield exposures of ∼1.34 to 4 IU/mL of either monoclonal RLYB212 or polyclonal RLYB211 were each effective at removing nearly all APLDQ+ platelets from circulation within 5 hours (Figure 2). Similar doses of these reagents were also effective at preventing alloimmunization, as determined by measuring antibody titers 2 and 3 weeks following exposure to murine HPA-1a+ platelets (Figure 3A-B; supplemental Figure 3, available on the Blood Web site). To assess the ability of prophylactic anti–HPA-1a antibody treatment to sustain prevention of alloimmunization to repeated immune challenges, a second round of prophylaxis with RLYB211 and APLDQ platelet transfusion was performed 21 days after the initial challenge. Again, doses projected to achieve 1 to 4 IU/mL of RLYB211 significantly reduced or completely prevented APLDQ alloimmunization (Figure 4A). Finally, female mice that had been exposed to APLDQ+ murine platelets in the presence of RLYB211, when bred with APLDQ+ males, gave birth to pups with platelet counts that were elevated in direct proportion to the dose of the antibody that had been given to the dam, with little protection at ∼0.25 IU/mL and nearly full protection at ∼4 IU/mL (Figure 4B). Anti-APLDQ maternal alloantibody titers in the dams (Figure 4C) and neonates (Figure 4D) showed similar dose-dependent reductions that corresponded inversely to neonatal platelet counts.
RLYB211 and RLYB212 induce clearance of circulating HPA-1a+ murine platelets. Platelets isolated from APLDQ+ C56BL/6 mice were labeled with the cell tracker dye CMFDA and were transfused (1 × 108 CMFDA-labeled platelets per mouse) into WT Balb/c female mice. One hour after transfusion, an irrelevant human control mAb (1.34 μg), normal human total IgG (2 mg), RLYB211, or RLYB212 at the indicated concentrations were introduced by tail vein injection. Blood samples were collected from the submandibular vein of the recipients following platelet transfusion but before antibody injection and at 5 and 24 hours post–antibody injection. The percentage of CMFDA-labeled platelets remaining in the circulation was determined by flow cytometry. (A,C) Representative flow-cytometric dot plots showing the presence of CMFDA-labeled APLDQ platelets remaining in circulation (box) over time. (B-D) The survival of transfused CMFDA-labeled APLDQ platelets in WT Balb/c female mice at 5 and 24 hours post–antibody injection. The survival of the transfused labeled platelets was calculated by the percentage of the remaining CMFDA+ platelets divided by the beginning percentage of CMFDA+ platelets. Data in panels B and D are the quantification of 5 separate experiments, shown as mean plus or minus standard error of the mean. N = 5 per group. ∗P < .05; ∗∗P < .01 vs normal human IgG as analyzed by the Mann-Whitney U test. nhIgG, normal human IgG; ns, not significant.
RLYB211 and RLYB212 induce clearance of circulating HPA-1a+ murine platelets. Platelets isolated from APLDQ+ C56BL/6 mice were labeled with the cell tracker dye CMFDA and were transfused (1 × 108 CMFDA-labeled platelets per mouse) into WT Balb/c female mice. One hour after transfusion, an irrelevant human control mAb (1.34 μg), normal human total IgG (2 mg), RLYB211, or RLYB212 at the indicated concentrations were introduced by tail vein injection. Blood samples were collected from the submandibular vein of the recipients following platelet transfusion but before antibody injection and at 5 and 24 hours post–antibody injection. The percentage of CMFDA-labeled platelets remaining in the circulation was determined by flow cytometry. (A,C) Representative flow-cytometric dot plots showing the presence of CMFDA-labeled APLDQ platelets remaining in circulation (box) over time. (B-D) The survival of transfused CMFDA-labeled APLDQ platelets in WT Balb/c female mice at 5 and 24 hours post–antibody injection. The survival of the transfused labeled platelets was calculated by the percentage of the remaining CMFDA+ platelets divided by the beginning percentage of CMFDA+ platelets. Data in panels B and D are the quantification of 5 separate experiments, shown as mean plus or minus standard error of the mean. N = 5 per group. ∗P < .05; ∗∗P < .01 vs normal human IgG as analyzed by the Mann-Whitney U test. nhIgG, normal human IgG; ns, not significant.
Administration of HPA-1a–specific antibodies prevents HPA-1a exposure-induced alloimmunization. (A-B) Female WT BALB/c mice either received 2 mg of normal human IgG, 0.25, 1.0, 4 IU/mL of RLYB211 (A) or 1.34 μg ZB007 (normal human IgG), or 1.2, 4.0, 12.0, 40.0 IU/mL of RLYB212 (B) 1 hour before transfusion of 1 × 108 APLDQ murine platelets. Blood was collected at the indicated time points, and the antibodies present that were reactive against APLDQ platelets were measured by flow cytometry. Results are reported as median fluorescence intensity (MFI) (mean ± standard error of the mean, n = 7-12 per group). Note the dose-dependent prevention of HPA-1a–induced alloimmunization, with nearly complete protection beginning at 1 IU/mL of polyclonal RLYB211 and at 4 IU/mL of monoclonal RLYB212. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs normal human IgG as analyzed by the Mann-Whitney U test. nhIgG, normal human IgG; ns, not significant.
Administration of HPA-1a–specific antibodies prevents HPA-1a exposure-induced alloimmunization. (A-B) Female WT BALB/c mice either received 2 mg of normal human IgG, 0.25, 1.0, 4 IU/mL of RLYB211 (A) or 1.34 μg ZB007 (normal human IgG), or 1.2, 4.0, 12.0, 40.0 IU/mL of RLYB212 (B) 1 hour before transfusion of 1 × 108 APLDQ murine platelets. Blood was collected at the indicated time points, and the antibodies present that were reactive against APLDQ platelets were measured by flow cytometry. Results are reported as median fluorescence intensity (MFI) (mean ± standard error of the mean, n = 7-12 per group). Note the dose-dependent prevention of HPA-1a–induced alloimmunization, with nearly complete protection beginning at 1 IU/mL of polyclonal RLYB211 and at 4 IU/mL of monoclonal RLYB212. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs normal human IgG as analyzed by the Mann-Whitney U test. nhIgG, normal human IgG; ns, not significant.
Repeat administration of HPA-1a–specific antibodies provides sustained protection from HPA-1a exposure-induced FNAIT. (A) Female WT BALB/c mice from Figure 3A that had been challenged 3 weeks earlier with APLDQ platelets in the presence or absence of RLYB211 were rechallenged at day 21 with 1 × 108 APLDQ platelets with or without prior tail vein injection of additional RLYB211 or nonbinding human IgG. Blood was collected at the indicated time points, and the maternal antibodies present that were reactive against APLDQ platelets were measured by flow cytometry. Results are reported as median fluorescence intensity (MFI) (mean plus or minus SEM, n = 8-10 per group). (B-D) BALB/c female mice that had been challenged twice with APLDQ platelets in the presence or absence of RLYB211 were then bred at day 35 with APLDQ males. Approximately 21 days later, pups were born and their platelet counts determined within 48 hours of birth (mean ± SEM, n = 44-69 per group) (B). APLDQ-reactive maternal antibody levels present in the dam (mean ± SEM, n = 6-9 per group) (C) and in the pups (D) (mean ± SEM, n = 44-69 per group) were then determined by flow cytometry. Platelet counts in pups born to female mice that had been treated with either no antibody or normal human IgG served as controls. Note the dose-dependent nature of protection, beginning at ∼1 IU/mL RYLB211. Significance was determined using the Mann-Whitney U test. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. nhIgG, normal human IgG; SEM, standard error of the mean.
Repeat administration of HPA-1a–specific antibodies provides sustained protection from HPA-1a exposure-induced FNAIT. (A) Female WT BALB/c mice from Figure 3A that had been challenged 3 weeks earlier with APLDQ platelets in the presence or absence of RLYB211 were rechallenged at day 21 with 1 × 108 APLDQ platelets with or without prior tail vein injection of additional RLYB211 or nonbinding human IgG. Blood was collected at the indicated time points, and the maternal antibodies present that were reactive against APLDQ platelets were measured by flow cytometry. Results are reported as median fluorescence intensity (MFI) (mean plus or minus SEM, n = 8-10 per group). (B-D) BALB/c female mice that had been challenged twice with APLDQ platelets in the presence or absence of RLYB211 were then bred at day 35 with APLDQ males. Approximately 21 days later, pups were born and their platelet counts determined within 48 hours of birth (mean ± SEM, n = 44-69 per group) (B). APLDQ-reactive maternal antibody levels present in the dam (mean ± SEM, n = 6-9 per group) (C) and in the pups (D) (mean ± SEM, n = 44-69 per group) were then determined by flow cytometry. Platelet counts in pups born to female mice that had been treated with either no antibody or normal human IgG served as controls. Note the dose-dependent nature of protection, beginning at ∼1 IU/mL RYLB211. Significance was determined using the Mann-Whitney U test. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. nhIgG, normal human IgG; SEM, standard error of the mean.
Discussion
FNAIT affects 1 in 1000 to 2500 live births in the United States and Europe each year. Unlike the more well-known HDN (or Rh syndrome), however, in which the overwhelming majority of cases occur in multigravida patients following alloimmunization from a prior pregnancy, 25% or more of alloimmunization events leading to FNAIT occur without warning during the gestation period of the first pregnancy.1,2 Also, unlike HDN, there are currently no approved treatments to prevent FNAIT from occurring.
Strategies to prevent alloimmunization to HPA-1a by inducing so-called antibody-mediated immune suppression, recently reviewed by Kjaer et al,26 were proposed nearly 10 years ago by Tiller and colleagues,15 who showed that injection of GPIIIa-specific antibodies is able to prevent GPIIb-IIIa–deficient mice from developing antibodies to a number of human platelet membrane glycoproteins. This animal model, however, was not alloantigen-specific as (1) the human platelets used for immunization could be HPA-1a or -1b homozygous; (2) the antibodies used to prevent alloimmunization did not themselves need to be HPA-1a–specific; (3) the antibodies generated were iso-, rather than allo-, antibodies; and (4) the model was not glycoprotein specific as it prevented antibodies from forming against both GPIIIa and GPIb (and we suspect many other human xenoantigens). As such, the GPIIb-IIIa–deficient mouse model is not able to adequately model the patient population at risk for FNAIT to support the preclinical efficacy and safety studies required to assess therapeutic candidates. The development of this alloantigen-specific APLDQ mouse model enables a true biallelic preclinical model to replicate the intended patient population of an HPA-1a− patient bearing an HPA-1a+ fetus. This is a crucial step in support of advancing therapeutic candidates into clinical trials aimed at management of this too-often serious alloimmune platelet disorder affecting fetuses and neonates.
The model, however, is not without limitations as naïve female mice require preimmunization with some form of APLDQ murine GPIIIa in order to generate HPA-1a–specific alloantibodies as they do not make them solely as a result of pregnancy (data not shown). We do not yet know whether previously unimmunized pregnant female mice are ignorant of, or tolerant to, the HPA-1a antigen presented by the fetus during the 3-week gestation period, which itself might be too short to enable female mice to generate an APLDQ-specific alloimmune response. Studies are underway to examine the mechanisms responsible for this limitation as they may yield important insights into how the maternal immune response to fetal alloantigens, in general, is regulated during pregnancy. Finally, the number of leukocytes present in the washed platelet product used to preimmunize female mice prior to mating them with APLDQ males is not controlled for in this model. Whether or not contaminating leukocytes are able to influence either the qualitative or quantitative nature of the maternal alloimmune antiplatelet response is not yet known.
Prophylactic treatment with passive transfer of low doses of anti-RhD IgG during pregnancy and at parturition has been safely and successfully used for >50 years to prevent RhD alloimmunization, with ∼99% efficacy rates. It is well established that effective prophylaxis with anti-RhD IgG requires that foreign antigen (D+) RBCs are eliminated rapidly and completely from circulation, and it has been estimated that effective opsonization requires that only ∼8% of RhD antigen on RBCs is bound.27 Interestingly, monoclonal anti-D antibodies, though in theory safer, less expensive, and more easily standardized, have shown great variability in their potency to both eliminate foreign antigen and prevent RhD alloimmunization in clinical trials.28 Growing data suggests that the mechanisms underlying this variability among anti-D mAbs may lie chiefly with the manufacturing process employed, in that the selection of cell line used for expression can greatly influence the composition of the glycan chains emanating from Asn297 within the Fc region of the mAb.29 In this study, we found that low doses of both the monoclonal antibody RLYB212 and the polyclonal antibody RLYB211 can induce rapid and complete elimination of HPA-1a+ murine platelets from circulation (Figure 2) and prevent alloimmunization (Figure 3). Low doses of RLYB211 also blocked development of FNAIT at concentrations predicted to opsonize only ∼10% of GPIIb-IIIa receptors (Figure 4), consistent with the threshold for efficacy established for polyclonal anti-RhD treatment. Importantly, because the half-life of RLYB211 in the mouse circulation is <24 hours (supplemental Figures 1 and 2), the major mechanism by which it exerts its suppressive effects is likely simple rapid antigen clearance so that an alloimmune response never has a chance to develop.
The ability to evaluate the relative effectiveness of newly developed, alloantigen-specific, immunotherapeutic reagents designed to prevent alloimmunization to the HPA-1a epitope, made possible by the availability of the first alloantigen-specific animal model of FNAIT, should facilitate clinical trials in humans aimed at management of this too-often serious alloimmune platelet disorder affecting fetuses and neonates. In this regard, in preliminary reproductive toxicology studies in which RLYB211 and RLYB212 were administered to pregnant WT C57BL6 mice bred to APLDQ sires, no drug-related toxicities have been observed in HPA-1a− dams or APLDQ+ fetuses or pups at the exposures found here to be effective at preventing alloimmunization (data not shown). Finally, a preliminary report in HPA-1a− healthy human volunteers found that a single IV dose of 1000 IU of RLYB211 is effective in driving the rapid elimination of 1 × 1010 HPA-1a+ platelets transfused into HPA-1a− volunteers, with near complete elimination within ∼2 hours.30 Because the effective circulating concentration of polyclonal anti–HPA-1a in these subjects was <0.5 IU/mL, these studies provide initial clinical proof of concept for the ability of low doses of anti–HPA-1a IgG to prevent alloimmunization in humans. Taken together, the combined preclinical and clinical data for both of these reagents provide support for safe and effective prophylactic use of anti–HPA-1a IgG in pregnant women at risk of FNAIT.
There are, of course, many other platelet membrane glycoprotein polymorphisms that can cause FNAIT in the human population31 and, depending on the region of the world in which they occur, can be of greater clinical importance than is HPA-1a/-1b. As such, novel immunotherapeutic regimens to treat or prevent FNAIT may not be limited to the HPA-1a/1b polymorphism as Xu and colleagues reported successful prenatal therapy of anti-CD36–mediated severe FNAIT in mice using a deglycosylated antibody specific for CD36.32 CD36 is a scavenger receptor found on the surface of many cell types, including platelets, monocytes, erythroblasts, capillary endothelial cells, and mammary epithelial cells.33,34 Because it is absent from the surface of platelets in 3% to 5% of Asians and Africans,35 CD36-deficient women in these populations are at risk of producing isoantibodies against this protein if exposed to it through transfusion or pregnancy. Xu et al took advantage of a strategy originally proposed by Bakchoul et al that employs a deglycosylated antigen-specific antibody generated by an enzymatic or genetic modification that abrogates recognition by Fc receptors without interfering with placental transport into the fetus.36 That therapeutic approach is fundamentally different from the one described in the present study as it relies on saturating concentrations of an effector-silent therapeutic antibody to outcompete maternal allo- or isoantibodies for binding to fetal platelets, thereby preventing in vivo destruction of antigen-positive platelets. Taken together, novel strategies such as these hold great promise for the future eradication of this too-often devastating alloimmune platelet disorder in humans.
In summary, these data establish that administration to mice of low doses of antibodies specific for the HPA-1a polymorphism prevents development of an alloimmune response triggered by exposure to HPA-1a+ platelets. The threshold effect for rapid and complete elimination of HPA-1a+ platelets is achieved at circulating anti–HPA-1a antibody concentrations estimated to bind as little as ∼10% of antigen, consistent with the clinical prophylactic treatment paradigm established for anti-RhD. These preclinical findings establish the proof of concept that administration of HPA-1a polyclonal- or monoclonal-specific antibodies below the threshold for clinical sequelae can effectively prevent anti–HPA-1a alloimmunization in pregnant women at risk of developing fetal/neonatal alloimmune thrombocytopenia.
Acknowledgments
This work was supported by grant R35-HL139937 (P.J.N.) from the National Heart, Lung, and Blood Institute of the National Institutes of Health and by a research grant to P.J.N. from Rallybio.
Authorship
Contribution: H.Z. designed and performed experiments, interpreted results, and wrote the paper; D.K.N. and D.S. designed experiments, interpreted results, and edited the paper; and P.J.N. designed experiments, interpreted results, and wrote the paper.
Conflict-of-interest disclosure: P.J.N. is a consultant for Rallybio, Inc. in the field of platelet immunobiology. D.S. is a research and development scientist employed by Rallybio, a clinical-stage biotechnology company headquartered in New Haven, CT. The remaining authors declare no competing financial interests.
Correspondence: Peter J. Newman, Blood Research Institute, Versiti Blood Center of Wisconsin, 8727 W. Watertown Plank Rd, Milwaukee, WI 53226; e-mail: pjnewman@versiti.org.
References
Author notes
All data associated with this study are available in the main text or the supplemental materials. Materials and mice are freely available to academic and not-for-profit investigators with appropriate material transfer agreements in place.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.