Key Points
Patients treated with next generation BTKi therapy acalabrutinib still see a >8 fold risk of ventricular arrhythmia and sudden death events.
Ventricular arrhythmias may be a class effect of BTKi therapy, and vigilance is needed.
Abstract
Acalabrutinib, a next-generation Bruton’s tyrosine kinase inhibitor (BTKi), associates with dramatic efficacy against B-cell malignancies. Recently, unexplained ventricular arrhythmias (VAs) with next-generation BTKi-therapy have been reported. Yet, whether acalabrutinib associates with VAs in long-term follow-up is unknown. Leveraging a large-cohort of 290 consecutive B-cell malignancy patients treated with acalabrutinib from 2014 to 2020, we assessed the incidence of VAs. The primary-endpoint was incident VA development (ventricular fibrillation, ventricular tachycardia, and symptomatic premature ventricular contractions). Probability-scores were assessed to determine likelihood of acalabrutinib-association. Incident rates as function of time-on-therapy were calculated. Weighted average observed incidence rates were compared with expected population rates using relative-risks. Absolute excess risk (AER) for acalabrutinib-associated VAs was estimated. Over 1063 person-years of follow-up, there were 8 cases of incident-VAs, including 6 in those without coronary disease (CAD) or heart failure (HF) and 1 sudden-death; median time-to-event 14.9 months. Among those without prior ibrutinib-use, CAD, or HF, the weighted average incidence was 394 per 100 000 person years compared with a reported incidence of 48.1 among similar-aged non–BTKi-treated subjects (relative risk, 8.2; P < .001; AER, 346). Outside of age, no cardiac or electrocardiographic variables associated with VA development. Collectively, these data suggest VAs may be a class-effect of BTKi therapies.
Introduction
Acalabrutinib is a novel next-generation Bruton’s tyrosine kinase inhibitor (BTKi) associated with dramatic efficacy against various B-cell malignancies and is now approved as indefinite therapy in those without intolerance or disease progression.1-5 In animal and clinical studies, acalabrutinib shows less cardiac atrial activity than ibrutinib, a first-generation BTKi.6,7 However, emerging reports of unexplained cases of incident ventricular arrhythmias (VAs) and sudden cardiac death with second (next)-generation BTKi therapy have been reported.8 With ibrutinib, similar insidious ventricular events were not appreciated until well after initial clinical use.9,10 Yet, whether this is also seen with next-generation BTKi therapy in long-term follow-up is unknown.
Study design
Leveraging data from a large and contemporary United States–based Comprehensive Cancer Center cohort of consecutive patients treated with acalabrutinib from 2014 to 2020 following institutional review board approval, we explored the rate of incident (first-ever) symptomatic VAs. Study patients included adults ≥18 years old, treated with acalabrutinib for a hematologic malignancy. Patients with incomplete medical records for the variables of interest or fewer than 7 days of acalabrutinib use were excluded (supplemental Figure 1, available on the Blood Web site). We manually searched all subject charts for incident VAs. Symptomatic premature ventricular tachycardia, sustained ventricular tachycardia (VT), and ventricular fibrillation were considered incident VAs.11 Arrhythmia episodes were graded using the Common Terminology Criteria for Adverse Events v5.0, followed by adjudication by independent cardiologists.11,12 VA-associated symptoms included chest pain, palpitations, dizziness, syncope, heart failure symptoms, and sudden cardiac death.
Our primary outcome was the development of incident symptomatic VAs following acalabrutinib initiation. The secondary outcome was the development of any symptomatic arrhythmia (supraventricular tachycardia [SVT] or VA). Follow-up began from acalabrutinib initiation. Univariable and multivariable Fine and Gray regression analyses accounting for competing risks were performed to determine the association between baseline covariates and outcomes. Survival analysis techniques were used to estimate the cumulative incidence of VAs. Person-year incidence rates for VA development on acalabrutinib were calculated. Descriptive statistics were used to summarize patient characteristics, using mean ± standard deviation or median (interquartile range) for continuous variables and frequency counts with percentages for categorical variables. Additionally, subgroup analysis among patients without prior/baseline myocardial infarction and/or left ventricular ejection fraction of <50% (systolic heart failure) was performed. Observed idiopathic VA rates were compared with expected incidence rates derived from published contemporary data from the Rochester Epidemiology Project, and ibrutinib-reported rates, using calculated relative risks (RRs).9,11 Absolute excess risk was calculated by subtracting the expected number of cases from the number of observed, multiplying by 100 000, and finally dividing by person years at risk. A Naranjo probability score was calculated for each event to determine the likelihood of acalabrutinib association, with a score of ≥5, suggestive of at least probable association.13
Results and discussion
Overall, 290 patients with median age of 63.9 ± 10.3 years (and 28.6% female, 58.9% hypertensive, 15.2% diabetic, 4.5% with myocardial infarction, 5.5% with heart failure) were treated with acalabrutinib for hematologic malignancies (supplemental Table 1). Most (89%) had chronic lymphocytic leukemia, and >95% (95.9%) had an Eastern Cooperative Oncology Group performance status of 0 to 1. Concurrent or prior anticancer therapy was used in 175 (60.3%), including 77 (26.6%) previously treated with ibrutinib; no patients received anthracyclines. The median duration of acalabrutinib use was 42 months.
Over a median follow-up of 42.3 months (range, 0.1-89), 10 patients developed symptomatic VAs (including 1 sudden death/ventricular fibrillation and 1 recurrent sustained VT), of which 80.0% (8) had at least probable association with acalabrutinib (Figure 1; supplemental Table 2). Twenty-nine developed any symptomatic arrhythmia (8 with non-AF SVTs). Among those with VAs of at least probable acalabrutinib association, the median time to event was 14.9 months (range, 1.1-55.8), whereas the median time to any arrhythmic event was 12.7 months (range, 0.5-70.3). Two patients required acalabrutinib discontinuation, and 1 required an implanted cardiac defibrillator for VT (supplemental Table 3). Over the 1063 person years of acalabrutinib exposure, the corresponding estimated 100 000 person-year VA incidence rate was 818.
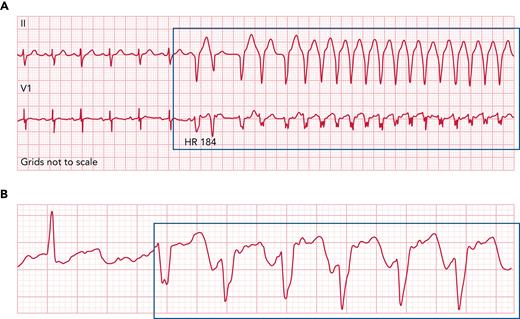
Representative ECG rhythm strips from patients with ventricular arrhythmias following acalabrutinib initiation. Representative ECG rhythm strip from a patient (A) with unexplained ventricular tachycardia episodes nearly 6 weeks after acalabrutinib initiation for chronic lymphocytic leukemia, as well as (B) from a patient with symptomatic premature ventricular contractions nearly 20 months after acalabrutinib initiation. Acalabrutinib dose was 100 mg twice daily, and symptoms included fatigue, palpitations, and shortness of breath. ECG, electrocardiogram; HR, heart rate.
Representative ECG rhythm strips from patients with ventricular arrhythmias following acalabrutinib initiation. Representative ECG rhythm strip from a patient (A) with unexplained ventricular tachycardia episodes nearly 6 weeks after acalabrutinib initiation for chronic lymphocytic leukemia, as well as (B) from a patient with symptomatic premature ventricular contractions nearly 20 months after acalabrutinib initiation. Acalabrutinib dose was 100 mg twice daily, and symptoms included fatigue, palpitations, and shortness of breath. ECG, electrocardiogram; HR, heart rate.
Outside of older age, no cardiovascular or electrocardiographic variables were found to be associated with VAs (supplemental Table 4). Yet, prior AF, prolonged corrected QT, and widened QRS complex, respectively, were associated with development of any arrhythmia (VA plus SVT). In a multivariable model, only prior AF was associated with arrhythmic events.
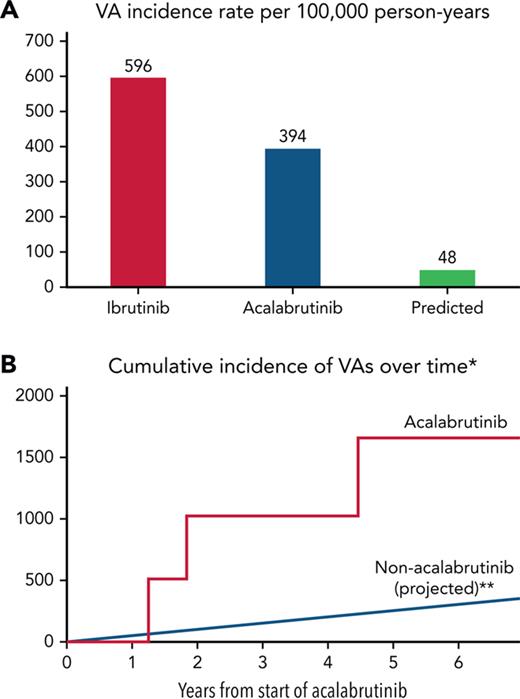
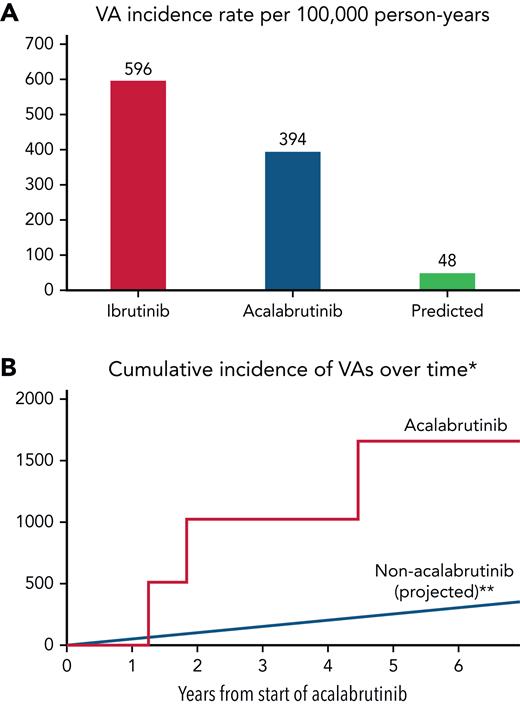
Furthermore, among those without baseline coronary disease and systolic heart failure, 6 patients had an incident VA event, corresponding to an estimated 100 000 person-year incidence rate of 685 (supplemental Figure 2). In those without prior ibrutinib treatment, the estimated person-year incidence rate was 393.9 (Figure 2). When compared with a reported ibrutinib-related VA incidence of 596 per 100 000 person years,9 this represented a RR of 0.66. Yet, when compared with a reported idiopathic VA incidence of 48.1 among similar nonacalabrutinib subjects,11 this still translated into an observed vs expected RR of 8.2 (P < .001) and absolute excess risk of 346.
Ventricular arrhythmia incidence rates in acalabrutinib users vs ibrutinib and non–BTKi-treated populations. VA crude (A) and cumulative (B∗∗) incidence rates in acalabrutinib users vs ibrutinib and non–BTKi-treated populations, respectively. ∗Those without prior ibrutinib or structural heart disease. ∗∗Assumes a linear event rate over time.
Ventricular arrhythmia incidence rates in acalabrutinib users vs ibrutinib and non–BTKi-treated populations. VA crude (A) and cumulative (B∗∗) incidence rates in acalabrutinib users vs ibrutinib and non–BTKi-treated populations, respectively. ∗Those without prior ibrutinib or structural heart disease. ∗∗Assumes a linear event rate over time.
In this evaluation of ventricular events after acalabrutinib initiation, nearly 3% developed a cardiotoxic VA event, including 1 with sudden death. In long-term follow-up, the excess risk of VA events was over eightfold higher than expected. This pattern remained, even after accounting in the presence or absence of structural heart disease and prior ibrutinib use. This observation is concerning, particularly given the rapid rise in acalabrutinib utilization and the potentially serious consequences of unrecognized cardiotoxic VA events.
Postmarket surveillance is pivotal to the recognition of unanticipated effects with many therapeutics. With ibrutinib, clinical and disproportionality analyses revealed signals of higher ventricular events not initially appreciated in clinical trials.9,10,14 Due to the confounding nature of VA events in older populations, particularly during times of stress, and seemingly isolated event presentations, appreciation of potential associations may be not be well recognized. Further, the potential association of BTKis with VAs was not known until after the initiation of early next-generation trials, including ELEVATE-RR.7,9,10 Within this study, all rhythm strips, electrocardiograms, and mobile cardiac monitors were manually adjudicated by independent cardiologists, which was not available within early trials. Although the exact mechanisms behind these events are not well understood, late sodium current–enhanced automaticity and enhanced fibrosis have been proposed.8,9,15 Limitations include VAs range in severity, and some intermittent baseline or postinitiation events were unrecognized. Some sudden death events were excluded due to overlap with other events (ie, infection) and thus not included in the final estimate. Further, some out-of-hospital cardiac events may have gone uncaptured despite extensive search.
In summary, acalabrutinib is associated with an increased risk of incident VA events in long-term follow-up. Given the anticipated increase in acalabrutinib use, clinicians should still monitor for VA symptoms and maintain a low threshold for cardiac workup. Further research targeting the mechanism(s) and prevention of ventricular arrhythmias among patients receiving BTKi therapies is needed.
Acknowledgments
The authors acknowledge and thank the patients and their families treated at the Ohio State University Comprehensive Cancer Center.
This work was supported in part by National Institutes of Health (NIH) National Cancer Institute (NCI) grants K23-CA178183 (J.W.) and R01-CA197870 (J.C.B. and J.W.), R35-CA197734 (J.C.B.), K12-CA133250 (D.A. and J.C.B.), and K23-HL155890 (D.A.). K.A.R. and J.W. were supported by scholar in clinical research grants from the Leukemia & Lymphoma Society (CDP 2331-20). D.A. was also supported by a Robert Wood Johnson Foundation (Harold Amos) American Heart Association Program grant. Support was also received from the D. Warren Brown Foundation, Four Winds Foundations, and the Connie Brown CLL Foundation. The manuscript’s content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: S.A.B., L.A., J.W., and D.A. contributed to concept and design; S.A.B., J.G., L.A., S.T.C., L.R., M.P., Q.Z., S.K., P.R., and D.A. contributed to acquisition, analysis, or interpretation of data; J.G., L.A., S.T.C., and D.A. contributed to drafting of the manuscript; S.T.C., M.P., and Q.Z. contributed to statistical analysis; S.A.B., J.C.B., J.W., and D.A. contributed to administrative, technical, or material support; S.A.B. and D.A. contributed to supervision; and all of the authors had full access to all of the data in the study, contributed to critical revision of the manuscript for important intellectual content, reviewed drafts of the manuscript, and approved the final version.
Conflict-of-interest disclosure: J.C.B. has received research funding and has consulted for Acerta Pharma and Pharmacyclics, Inc. F.A. has received research funding from Innate Pharma and Pharmacyclics and provided consulting services to Gilead Sciences; Pharmacyclics, Inc; Janssen; Abbvie; Sunesis; AstraZeneca; Genentech; and Novartis Oncology and served on the speakers bureau of Abbvie and AstraZeneca. K.A.R. received research funding from Genentech, Novartis, Janssen, and AbbVie and consulted for Pharmacyclics, AstraZeneca, Genentech, Abbvie, Innate Pharma, and Beigene. A.K. has provided consulting services for Abbvie, Beigene, Bristol-Myers Squibb, and Janssen. J.W. received research funding from Abbvie, Pharmacyclics, Janssen, Acerta, Loxo, Karyopharm, and Morphosys and has consulted for Janssen and Pharmacyclics. M.G. is consulting and serving on a data safety and monitoring committee for Ascerta related to acalabrutinib. The remaining authors declare no competing financial interests.
Correspondence: Daniel Addison, Division of Cardiovascular Medicine, Davis Heart & Lung Research Institute, 473 West 12th Ave, Suite 200, Columbus, OH 43210; e-mail: daniel.addison@osumc.edu.
References
Author notes
∗S.A.B., J.G., L.A., S.T.C., and D.A. contributed equally to this study
Presented in abstract form at the annual scientific sessions meeting of the American Heart Association, Chicago, IL, 6 November 2022.
Send data sharing requests via email to the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

Comments
Response to Comments: Ventricular Arrhythmias and Sudden Death Events following Acalabrutinib Initiation
We acknowledge the comments presented by Drs. Smith, Sharman, Ghia, and Seymour, regarding our original research manuscript, Bhat, et al., Blood1, including questions on the relevance of frequent and symptomatic premature ventricular contractions (PVCs) among acalabrutinib-treated cancer patients.
The role of PVCs has been an area of previous intense focus and evaluation in cardiovascular medicine.2-4 Available systematic epidemiologic data suggest symptomatic PVCs, particularly left-sided origin, portend worse outcomes across multiple populations.2-4 Recent data have established associations between symptomatic PVCs and/or high PVC burden and increase in the risk heart failure, sustained ventricular arrhythmia-(VA), and sudden or cardiovascular-death, even among non-cancer patients without baseline cardiovascular disease-(CVD).2-4
However, similar unbiased data are largely unavailable in cancer populations. This includes evaluation using: 1). longer-term electrocardiographic (ECG)-monitoring, 2). systematic symptom-triggered ECG evaluation, and 3). evaluation by blinded cardiovascular experts.2-4 The only available prospective study using systematic longer-term (≥24 hours of continuous) ECG-monitoring of patients receiving ibrutinib, revealed surprisingly high-rates of incident atrial fibrillation reaching 38%.5 Yet, no such study exists for patients receiving next-generation BTKIs, and there are no systematic data on the incidence or burden of VAs. These gaps present serious questions for the cardiovascular and hematologic communities.
The current study raises the antenna for awareness of the potential risk of VAs and sudden-death events with BTKIs, even beyond ibrutinib. This is particularly enforced by recognition of the persistence of the relationship between acalabrutinib-use and VA-risk, even after accounting for structural heart-disease (prior myocardial-infarction and heart failure- traditional VA-risk factors2-4), with relative-risk of 8.2 (P<0.001).1 Notably, the link between acalabrutinib and VA-susceptibility was confirmed in independent animal-models.5
We believe our systematic approach provides enhanced understanding of potential CVD effects of BTKIs. Although these patients are affected by cancer, a better understanding of true CVD effects and impact of novel treatments on long-term outcomes is still needed.
Disclosures:
This work was supported in part by an NIH P50-CA140158 grant. Dr. Fradley received grant support from Medtronic and AstraZeneca and provides consulting services to Abbvie, AstraZeneca, Johnson and Johnson, Myovant, Pfizer and Zoll. Dr. Kittai is supported by the ASCO Career Development Award, receives research funding from AstraZeneca, and has consulted for AstraZeneca, Abbvie, Beigene, BMS, Eli-Lilly, Janssen, and KITE. Dr. Rogers received research funding from Genentech, AbbVie, Janssen, and Novartis, has consulted for AstraZeneca, Janssen, Pharmacyclics, AbbVie, Genentech, Beigene, and LOXO@Lilly, and is a scholar in clinical research of the Leukemia & Lymphoma Society (CDP 2331-20). Dr. Woyach was supported by R01-CA197870, R01-CA250503, R01-CA177292, R01-CA192928 and scholar in clinical research grants from the Leukemia & Lymphoma Society (CDP 2331-20); and received research funding from Abbvie, Pharmacyclics, Janssen, Acerta, Loxo, Karyopharm, and Morphosys, and has consulted for Janssen and Pharmacyclics. Dr. Addison is supported by NIH grant numbers K23-HL155890 and R01-HL170038, and an American Heart Association‐Robert Wood Johnson Foundation Program grant. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References:
1. Bhat SA, Gambril J, Azali L, Chen ST, Rosen L, Palettas M, Wiczer TE, Kalathoor S, Zhao Q, Rogers KA, Kittai A, Grever M, Awan F, Ruz P, Byrd JC, Woyach J, Addison D. Ventricular arrhythmias and sudden death events following acalabrutinib initiation. Blood. 2022;140(20):2142-2145.
2. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Ventricular Ectopy as a Predictor of Heart Failure and Death. J Am Coll Cardiol. 2015;66(2):101-9.
3. Kim YG, Choi YY, Han KD, Min KJ, Choi HY, Shim J, Choi JI, Kim YH. Premature ventricular contraction increases the risk of heart failure and ventricular tachyarrhythmia. Sci Rep. 2021;11(1):12698.
4. Baptiste F, Cautela J, Ancedy Y, Resseguier N, Aurran T, Farnault L, Escudier M, Ammar C, Gaubert M, Dolladille C, Barraud J, Peyrol M, Cohen A, Paganelli F, Alexandre J, Ederhy S, Thuny F. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart. 2019;6(1):e001049.
5. Tarnowski D, Feder A-L, Trum M, Kreitmeier K-G, Stenel L, Maier LS, Sag CM. Ibrutinib impairs IGF-1-dependent activation of intracellular Ca handling in isolated mouse ventricular myocytes. Front. Cardiovasc. Med. 2023;10:1190099.
This ain’t the study for the broken-hearted
In addition, event risks were compared to general population data instead of age-matched patients with similar disease and baseline cardiovascular risk factors (1). The patient population reported by Bhat et al was treated in a US-based university practice setting and is not representative of the typical CLL population compared to larger registry analyses (2,3). The cohort analyzed consisted of predominantly elderly male patients with many comorbidities and multiple concomitant medications, factors that are known to increase the risk of cardiovascular events generally, and PVCs specifically (1,4). In a large (N=15,792) cross-sectional population-based study, PVCs were documented in >6% of those aged 45–64 years, with increasing age and male sex identified as risk factors (4). Overall, the analysis by Bhat et al is limited in its ability to establish causality or class effect in the absence of a prospective, comparative study, and there are insufficient data to point to an increased risk of VA or sudden death with acalabrutinib. Further evaluation of the question in large populations with appropriately matched controls is warranted.
1. Bhat SA, Gambril JA, Azali L, et al. Ventricular arrhythmias and sudden death events following acalabrutinib initiation. Blood. 2022;140(20):2142-2145.
2. Mato A, Nabhan C, Kay NE, et al. Real-world clinical experience in the Connect chronic lymphocytic leukaemia registry: a prospective cohort study of 1494 patients across 199 US centres. Br J Haematol. 2016;175(5):892-903.
3. Mato A, Nabhan C, Lamanna N, et al. The Connect CLL Registry: final analysis of 1494 patients with chronic lymphocytic leukemia across 199 US sites. Blood Advances. 2020;4(7):1407-1418.
4. Simpson RJ, Jr., Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2002;143(3):535-540.
A Shot to the Heart- but who's to blame?
Only 8 ventricular arrhythmias deemed related to acalabrutinib occurred, in a cohort with a median age of 64-- of whom 59% had HTN and at least 25% received antiarrhythmic agents at baseline—with 42 months of follow-up. It appears that most were symptomatic PVC’s, and the severity or grading of these events is not known. The only cardiac sudden death was in an 85 year-old, and 6 of 8 patients with VA were able to continue acalabrutinib; only one had paroxysmal VT diagnosed requiring an implanted defibrillator. As others have suggested (2), symptomatic PVC’s may not comprise a clinically significant entity; PVC’s can be seen in 6% of the population on a given 2-minute EKG (3).
The suggestion of a higher rate of VA than the general population is based on estimates from the Rochester Epidemiology Project, but a better comparator would be aged-matched CLL pts receiving non-BTK therapies. Differences in follow-up methods and scrutiny of patients with hematologic cancers; their overall health and supplemental medications; and factors directly related to CLL intersecting with cardiac risk (anemia, prior antineoplastic therapy) may mean that treated CLL populations are not comparable to the general population in key respects.
In the present report, the median time to VA event was 14.9 months compared to a report for ibrutinib in which time from start to ventricular fibrillation or tachycardia was 65 days. (5) While a cumulative dose effect of acalabrutinib are possible, this could also suggest enhanced case-finding of expected or random events, with long follow-up.
In modern prospective trials of BTKi, scrutiny of cardiac safety is high and rightfully so based on the known association with atrial fibrillation. Nonetheless, prospective trials in first line CLL and relapsed/refractory CLL found no ventricular arrythmias with acalabrutinib. (5,6) A pooled analysis of 762 trial patients showed a low incidence of new-onset cardiac AE, and no sudden deaths with acalabrutinib. (7) These prospective trial data must be considered in light of the data by Bhat and colleagues, and more importantly, when evaluating the risk/benefit ration of BTKi therapy for a given patient.
Overall, suggesting that VA represent a “class effect” of BTKi paves over likely differences between these agents, and is not useful when assessing the therapeutic index of an agent or monitoring therapy with a given patient. While post-marketing studies are critical, careful selection of endpoints, and an appropriator comparator, are necessary before concluding that VA risk with acalabrutinib is either elevated or clinically important.
1. Bhat, Ventricular Arrhythmias and Sudden Death Events following Acalabrutinib initiation. Blood 2022; blood.2022016953. doi: https://doi.org/10.1182/blood.2022016953
2. Hill, Brian. August 3, 2022. @BrianHillMD_PhD. Tweet/Twitter.com
3. Simpson Am Heart J. 2002;143(3):535.
4. Lampson Blood 2017 May 4;129(18):2581-2584.
5. Sharman Lancet 2020 Apr 18;395(10232):1278-1291
6. Byrd J Clin Oncol. 2021 Nov 1;39(31):3441-3452
7. Brown Haematologica 2022 Jun 1;107(6):1335-1346