TO THE EDITOR:
In most patients with multiple myeloma, the neoplastic plasma cells produce a monoclonal immunoglobulin, which can be measured in the serum and urine and serves as a biomarker to aid diagnosis and assess response. However, 1% to 3% of patients produce no detectable paraprotein and are described as having nonsecretory multiple myeloma (NSMM).1-5 A further 2% to 3% of patients have insufficient levels of paraprotein production for reliable serological monitoring of their treatment response5,6 and are described as having nonmeasurable oligo-secretory multiple myeloma (OSMM) (paraprotein <10 g/L, Bence Jones protein <200 mg/24 h, and involved serum free light chain [iFLC] <100 mg/L4). The gold standard for monitoring patients with nonmeasurable myeloma (NMM) is serial bone marrow biopsies4 but this is an invasive and often painful procedure. In real-world practice, many patients with NMM are monitored with serial imaging. However, there are limitations in availability and repeated exposure to high doses of radiation with serial positron emission tomography-computed tomography scans. Patients with NMM are challenging to diagnose and monitor and are frequently excluded from clinical trials, limiting their access to novel agents.
Mass spectrometry (MS) methodologies are emerging as a new and more sensitive way to monitor paraprotein levels in serum.7-12 They have recently been approved by the International Myeloma Working Group for use in lieu of immunofixation13 and have the advantage that they can quantitate low-level paraproteins.7 Preliminary studies have reported that the limit of detection for matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS is <10 mg/L.14 In this study, we investigated how many patients with NSMM have detectable paraprotein production using MALDI-TOF MS and the suitability of MS for monitoring patients with NMM.
MS analysis was performed on all available presentation and follow-up samples from patients with NSMM (immunofixation negative and iFLC <100 mg/L) and OSMM from during induction and up to day+100 after autologous stem cell transplantation (ASCT) from patients treated in the Myeloma XI trial. The baseline characteristics of these patients are listed in Table 1. The methods for the MS analysis have been described previously.7,14,15 Paraprotein detection rates were compared with the serum protein electrophoresis (SPE), immunofixation, and Freelite results.
Twenty of 22 (90.9%) patients with NSMM had detectable paraprotein by MS at presentation (example spectra in Figure 1). Seven of 22 (31.8%) had just a monoclonal FLC detectable with no detectable intact immunoglobulin paraprotein; all of these patients presented with an abnormal serum FLC ratio (sFLCr) (iFLC, 16.5-90.3 mg/L). Seven of 22 (31.8%) patients had an intact immunoglobulin paraprotein with no detectable monoclonal FLC. Three of 7 (42.9%) of these patients had an abnormal sFLCr (iFLC, 25.8-46.6 mg/L). The light chain isotype of the intact immunoglobulin paraprotein was the same as indicated by the skewed sFLCr in 2/3 (66.7%) patients. Six of 22 (27.3%) patients had both a detectable monoclonal intact immunoglobulin and monoclonal FLC. The monoclonal FLC were of the same isotype and had the same mass-to-charge ratio (m/z) as the light chain component of the intact immunoglobulin paraprotein in 3/6 (50%) patients, indicating they are derived from the same clone. Two of 3 (66.7%) had a normal sFLCr. Three of 6 (50%) patients had monoclonal FLC and a second intact immunoglobulin paraprotein with the opposite iFLC identified by MS. These patients all had abnormal sFLCr (iFLC, 33.1-87.7 mg/L). Both patients with no detectable paraprotein by MS had immune paresis, normal serum FLC levels, and sFLCr.
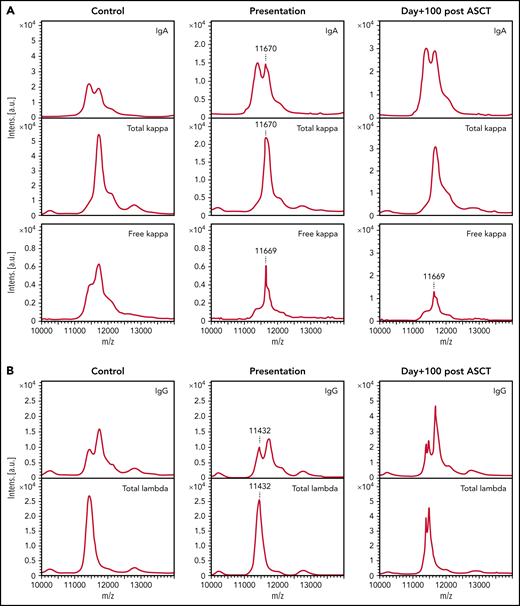
Paraproteins are detectable by MALDI-TOF MS in the sera of the majority of patients with NSMM and the unique m/z can be used to track the paraprotein through treatment. (A) A 0.086 g/L IgA κ paraprotein that was detectable in the presentation sample of a patient with NSMM (immunofixation negative, IgG 7.31 g/L, IgA 4.1 g/L, IgM 0.8 g/L, serum free κ 79.4 mg/L, serum free λ 22.7 mg/L, sFLCr 3.50) at m/z 11 670 and monoclonal κ FLC were detectable at m/z 11 669 for the doubly charged light chains, respectively. At day+100 after ASCT, the sFLCr had normalized (serum free κ 22.7 mg/L, serum free λ 14.12 mg/L, sFLCr 1.60) but MS detected residual monoclonal κ FLC with the same m/z as detected in the baseline sample. (B) A 0.14 g/L IgG λ monoclonal protein detectable by MS at m/z 11 432 for the doubly charged light chain in the presentation sample from a patient with NSMM (immunofixation negative, IgG 5.40 g/L, IgA 0.5 g/L, IgM 0.39 g/L, serum free κ 5.46 mg/L, serum free λ 6.08 mg/L, sFLCr 0.90). At day+100 after ASCT oligoclonal peaks are present in the IgG and λ spectra but no residual monoclonal protein is detectable at m/z 11 432.
Paraproteins are detectable by MALDI-TOF MS in the sera of the majority of patients with NSMM and the unique m/z can be used to track the paraprotein through treatment. (A) A 0.086 g/L IgA κ paraprotein that was detectable in the presentation sample of a patient with NSMM (immunofixation negative, IgG 7.31 g/L, IgA 4.1 g/L, IgM 0.8 g/L, serum free κ 79.4 mg/L, serum free λ 22.7 mg/L, sFLCr 3.50) at m/z 11 670 and monoclonal κ FLC were detectable at m/z 11 669 for the doubly charged light chains, respectively. At day+100 after ASCT, the sFLCr had normalized (serum free κ 22.7 mg/L, serum free λ 14.12 mg/L, sFLCr 1.60) but MS detected residual monoclonal κ FLC with the same m/z as detected in the baseline sample. (B) A 0.14 g/L IgG λ monoclonal protein detectable by MS at m/z 11 432 for the doubly charged light chain in the presentation sample from a patient with NSMM (immunofixation negative, IgG 5.40 g/L, IgA 0.5 g/L, IgM 0.39 g/L, serum free κ 5.46 mg/L, serum free λ 6.08 mg/L, sFLCr 0.90). At day+100 after ASCT oligoclonal peaks are present in the IgG and λ spectra but no residual monoclonal protein is detectable at m/z 11 432.
Seventeen of 22 (77.3%) patients were transplant eligible. Follow-up samples were available for MS testing from: 15/22 (68.2%) patients from after cycle 1; 11/22 (50%) patients from after induction; 5/17 (29.4%) patients from day+42 after ASCT and 8/17 (47.1%) from day+100 after ASCT. Overall, 20/39 (51.3%) of the follow-up samples had residual paraprotein detectable by MS (supplemental Table 1 available on the Blood Web site).
Dilutions of 2 samples from patients with NSMM into polyclonal serum (IgG 4.7 g/L, IgA 1.0 g/L, IgM 0.49 g/L, serum free κ 4.99 mg/L, and serum free λ 7.35 mg/L) were performed to assess the utility of MS for assessing response in patients with NSMM. Residual monoclonal FLC were detectable following 1:20 dilution of a sample with 48 mg/L serum free κ at presentation (supplemental Figure 1), demonstrating patients with iFLC <100 mg/L can have measurable disease using MS. However, no residual paraprotein was detectable following 1:2 dilution of a sample with 2 small IgA paraproteins (total paraprotein concentration 0.01 g/L), indicating the paraproteins detected in some patients may be useful as a marker of disease activity but are too small to be used in isolation for accurate response assessment.
Samples from 25 patients with OSMM were available for MS testing. Paraprotein of the same isotype as identified by immunofixation was detectable by MS in 25/25 (100%) samples. Thirty-eight follow-up samples from during induction chemotherapy or within 3 months after ASCT were available for MS testing (supplemental Table 1). Nineteen of 19 (100%) patients had residual paraprotein detectable by MS after cycle 1, including 1 patient who had no residual paraprotein detectable by immunofixation and a normal sFLCr. Residual paraprotein was detectable by SPE and/or immunofixation in 8/11 (72.7%) patients after induction. Residual paraprotein was detected by MS in all of these patients and 1 patient who was immunofixation negative with a normal sFLCr (0.12 g/L residual IgG κ paraprotein detectable by MS). Twelve of 25 (48.0%) patients were transplant eligible. Two of 4 (50.0%) of samples from day+42 after ASCT had residual paraprotein detectable by SPE and 1/4 (25.0%) patients had residual paraprotein only detectable by immunofixation. Residual quantifiable paraprotein was detectable by MS in all of these samples (paraprotein concentrations 0.23-0.97 g/L). One of 4 (25.0%) samples from day+100 after ASCT had residual paraprotein detectable by SPE. MS detected residual paraprotein in this sample and another patient who was immunofixation negative (paraprotein too small for accurate quantification by MS).
Six samples from patients with OSMM (paraprotein levels 2.55-9.3 g/L) were diluted 1:2, 1:10, and 1:20 into polyclonal serum to assess the suitability of MS for assessing response. Two monoclonal proteins spiked into polyclonal serum at 1 g/L were also analyzed. Residual paraprotein with the same isotype and m/z was detectable in all samples, indicating that MS could be used to assess a very good partial response.
Nonmyeloma-related conditions, including renal impairment and infection, can affect FLC levels, resulting in variable polyclonal FLC levels, making result interpretation difficult. Treatment-related immune suppression can also cause reduced levels of the uninvolved FLC, creating an abnormal sFLCr in the absence of residual disease.16 In this study, MS detected residual monoclonal FLC in patients in whom the sFLCr has normalized and excluded residual monoclonal FLC in patients with abnormal ratios resulting from treatment-related immune suppression, demonstrating that measurement of the monoclonal FLC specifically offers more accurate assessment of low-level residual disease.
A potential limitation of MS is that myeloma clones can reduce their immunoglobulin production during disease progression from dedifferentiation. Bone marrow assessments and imaging studies should therefore be included in MS studies to confirm the complete response and exclude nonimmunoglobulin producing residual disease when MS negativity is achieved.
In keeping with previous immunohistochemical and flow cytometry studies that have demonstrated that most patients with NSMM have evidence of paraprotein production,17,18 we found detectable paraprotein by MS in 91% of patients with NSMM. This indicates that most patients currently described as having NSMM have low-level paraprotein secretion rather than nonproduction or nonsecretion. MS assays should enable a significant proportion of patients with NMM to be defined as having measurable disease by MS leading to better monitoring of disease and allowing inclusion of such patients into future clinical trials. Future studies are needed to determine the criteria for measurable disease using MS assays.
The study was approved by the national ethics review board (National Research Ethics Service, London, UK), institutional review boards of the participating centers, and the competent regulatory authority (Medicines and Healthcare Products Regulatory Agency, London, UK), and was undertaken according to the Declaration of Helsinki and the principles of Good Clinical Practice as espoused in the Medicines for Human Use (Clinical Trials) Regulations. All patients provided written informed consent.
Acknowledgments
The Binding Site Ltd provided the reagents and instrumentation to perform the mass spectrometry analysis. The serum samples for mass spectrometry analysis were supplied UK Myeloma Trials Management Group. Primary financial support for Myeloma XI was from Cancer Research UK (C1298/A10410). Unrestricted educational grants from Celgene Corporation, Amgen, and Merck Sharp and Dohme, and funding from Myeloma UK, supported trial coordination and laboratory studies.
Authorship
Contribution: H.V.G. and G.P. designed the study; M.T.D. provided samples for mass spectrometry analysis; H.V.G. and N.W. performed the mass spectrometry analysis of the samples; H.V.G. and N.W. analyzed the mass spectrometry results; H.V.G., G.P., and M.C. wrote the manuscript; and all authors reviewed the final manuscript before submission for publication.
Conflict-of-interest disclosure: H.V.G. has received sponsorship for her PhD tuition fees from The Binding Site Ltd. G.P. has provides consultancy services for The Binding Site Ltd, Takeda, Amgen, Bristol Myers Squibb, Gilead, and Janssen. M.C. began working for Bristol Myers Squibb June 2021. M.T.D. provides consultancy services for Abingdon Health. G.C. provides consultancy services for Janssen, Takeda, Amgen, Bristol Myers Squibb, Sanofi, Oncopeptides, Karyopharm, Pfizer, and Roche. G.C. has received research funding from Janssen, Takeda, Amgen, and Bristol Myers Squibb. D.C. has received research funding from Bristol Myers Squibb, Merck Sharpe, and Takeda. M.K. provides consultancy services for AbbVie, Bristol Myers Squibb, Janssen, GlaxoSmithKline, Karyopharma, Pfizer, Amgen, Seattle Genetics, and Takeda. G.H.J. provides consultancy services for Amgen, GlaxoSmithKline, Johnson & Johnson, Takeda, and Bristol Myers Squibb; has received research funding from Takeda and Bristol Myers Squib; and has received honoraria from Sanofi. S.H., S.N., and N.W. are employed by The Binding Site.
Correspondence: Guy Pratt, Department of Haematology, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom; e-mail: guy.pratt@uhb.nhs.uk.
The online version of this article contains a data supplement.