Abstract
Many congenital or acquired nonmalignant diseases (NMDs) of the hematopoietic system can be potentially cured by allogeneic hematopoietic cell transplantation (HCT) with varying types of donor grafts, degrees of HLA matching, and intensity of conditioning regimens. Unique features that distinguish the use of allogeneic HCT in this population include higher rates of graft failure, immune-mediated cytopenias, and the potential to achieve long-term disease-free survival in a mixed chimerism state. Additionally, in contrast to patients with hematologic malignancies, a priority is to completely avoid graft-versus-host disease in patients with NMD because there is no theoretical beneficial graft-versus-leukemia effect that can accompany graft-versus-host responses. In this review, we discuss the current approach to each of these clinical issues and how emerging novel therapeutics hold promise to advance transplant care for patients with NMDs.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an established curative treatment most commonly used to treat malignant hematologic diseases.1 To date, allogeneic HCT has also long been the only curative option for patients with many life-threatening, congenital or acquired nonmalignant diseases (NMDs) involving the hematopoietic system.2 Robust clinical experience and long-term follow-up supports the use of allogeneic HCT in managing bone marrow (BM) failure syndromes,3,4 hemoglobinopathies,5,6 primary immunodeficiency diseases,7,8 inherited metabolic disorders,9 and autoimmune diseases10 (Table 1). Disease and transplant considerations that can pose unique challenges differentiate the application of allogeneic HCT to NMD compared with the treatment of hematologic malignancies. In this review, we highlight these challenges and describe how recent and future advances hold the potential to overcome current barriers and improve allogeneic HCT outcomes for patients with NMDs.
Transplant considerations for nonmalignant conditions
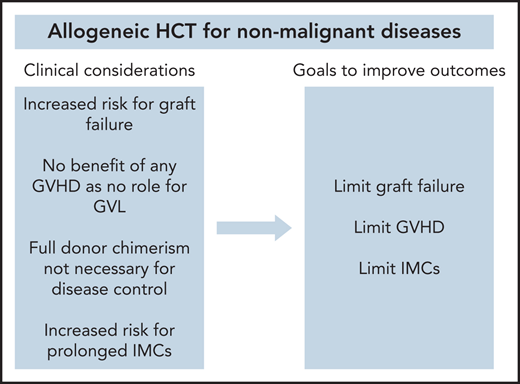
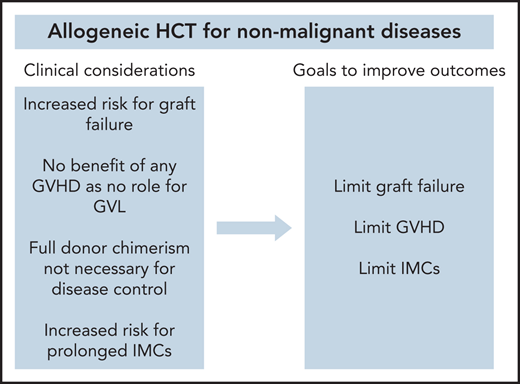
There are multiple clinical considerations that distinguish the use of allogeneic HCT for NMDs from its use for hematologic malignancies (Table 1). First, higher rates of primary and secondary graft failure have been observed after allogeneic HCT in patients with NMD compared with patients with acute leukemia (relative risk, 3.32; P < .01).11 There are multiple factors that possibly contribute to this issue. Reduced intensity conditioning (RIC) and nonmyeloablative (NMA) conditioning regimens have been used more regularly for select diseases in order to avoid both the short- and long-term toxicity associated with myeloablative conditioning (MAC) regimens, most notably in Fanconi anemia (FA) patients that have a DNA repair defect and hence inherent sensitivity to such.3 However, with lower doses of chemotherapy or radiation, a competent recipient immune system may persist and cause or facilitate graft rejection. In the absence of prior chemotherapy used to treat an underlying hematologic malignancy, for many of the NMDs such as those with inherited metabolic disorders, the host immune system that have graft rejection capabilities and BM hematopoietic and stromal cell compartments that harbor hematopoietic stem and progenitor cells (HSPCs) can compete with the donor graft would be largely unperturbed. Under these conditions, host HSPCs and immune cells may dominate repopulation in the recipient. Furthermore, patients with hemoglobinopathies who have received sufficient numbers of blood transfusions before transplant and have become immunized with donor-specific antibodies may more likely reject their allograft.12 Finally, BM is considered to be the standard graft source for many NMD transplant indications, given its association with lower rates of GVHD compared with peripheral blood stem cells.13 However, BM grafts contain lower CD34+ and total nucleated cell doses, which has been shown to be a significant risk factor for graft failure.11
Second, there is no potential benefit associated with the development of graft-versus-host disease (GVHD) in patients with NMDs. A core principle of allogeneic HCT in malignancies is that the curative effect is largely dependent on donor immune cells that eliminate residual malignant cells that persist after conditioning, conventionally referred to as the graft-versus-leukemia (GVL) effect.14 Thus, for patients with hematologic malignancy undergoing allogeneic HCT, efforts to minimize GVHD must also have a goal of preserving the GVL effect.15 Because there is no benefit for GVL in NMDs, more stringent GVHD prevention approaches are required.
Third, immune-mediated cytopenias (IMCs), including hemolytic anemia, thrombocytopenia, and/or neutropenia, are known to occur at a high incidence for patients with inherited metabolic disorders following allogeneic HCT.16 Although the etiologies of IMCs in patients with NMD have not been fully elucidated, interactions between the donor and recipient immune systems are thought to trigger these cytopenias, which may be further driven by abnormal immune-mediated reactions because of lysosomal dysfunction that leads to the excessive accumulation of storage disease substrates or end-products that may be seen as “foreign” by donor cells.17 Even outside of inherited metabolic disorders, a higher incidence of IMCs after allogeneic HCT have been observed with other NMDs compared with hematologic malignancies.16
Finally, full donor chimerism is not required to achieve therapeutic efficacy in NMDs. Typically, allogeneic HCT recipients experience full donor chimerism (>95% of cells are of donor origin), with low or falling chimerism being indicative of graft failure or relapse.18 Multiple studies in patients with hematologic malignancy have suggested the rapid full donor chimerism is associated with lower relapse rates,19-21 and interventions such as adjustment of immunosuppression or cellular therapies are often used to achieve such. Rarely, subsets of such patients never reach full donor chimerism but remain as mixed chimerism (5%-95% cells of donor origin). In NMDs, where complete replacement of recipient hematopoiesis is not always necessary to improve the underlying disease state, mixed chimerism may be sufficient to achieve a durable disease response with low rates of GVHD.22,23
Approaches to enhance allogeneic HCT for NMDs
To augment the success of allogeneic HCT for NMDs, novel approaches should be incorporated into transplant platforms that can limit: (1) graft rejection; (2) GVHD; and (3) IMCs. Conditioning regimens, graft sources, and GVHD immune prophylaxis regimens are three essential components of allogeneic HCT that will be prime targets for innovation (Table 2).
Conditioning regimens
We will briefly review the more widely used conditioning regimens for treating patients with representative hematopoietic, immune, or metabolism disorders before discussing new strategies to optimize myelosuppression and immunosuppression without the toxicities of conventional chemotherapies (Figure 1).
BM failure syndromes
RIC for patients with BM failure frequently consist of low-dose cyclophosphamide, fludarabine, and busulfan, and/or low-dose irradiation.24 Patients with FA and dyskeratosis congenita pose a unique challenge because of hypersensitivity to DNA alkylating agents and irradiation,25,26 necessitating reduced cyclophosphamide, a DNA alkylating agent, and/or radiation doses to decrease the risk of early toxicity (FA) and late secondary malignancies (both). Although total body irradiation (TBI) doses can be reduced to 300 cGy, further reduction has resulted in an increased incidence of graft rejection.27 Instead, fludarabine and alemtuzumab (anti-CD52 monoclonal antibody [mAb]) are commonly included to suppress graft rejection. A prospective multi-institutional study of alternative donor allogeneic HCT in patients with FA using low-dose busulfan, cyclophosphamide, fludarabine, rabbit anti-thymocyte globulin (ATG) and T-cell depleted grafts showed all patients younger than 10 years of age at allogeneic HCT survived and none developed severe acute GVHD.28 A similar trial for patients with dyskeratosis congenita is ongoing (NCT01659606).
Hemoglobinopathies
Stable donor engraftment after RIC or NMA allogeneic HCT can be difficult to obtain in patients with hemoglobinopathies who have an intact immune system and HSPC content. Patients with thalassemia or sickle cell disease (SCD) are at higher risk of graft failure even when MAC containing busulfan or cyclophosphamide are used. Treosulfan has been introduced as a lower toxicity substitute for busulfan; however, a third agent, thiotepa, is often needed to reduce the frequency of mixed chimerism when used in the context of hemoglobinopathies, where there is more active marrow function.29,30 Adding thiotepa to the previously tested RIC, consisting of alemtuzumab, fludarabine, and melphalan, has also been used successfully in umbilical cord blood transplantation for patients with SCD.31 Furthermore, haploidentical HCT with posttransplantation cyclophosphamide (PTCy) and thiotepa has been shown to improve donor engraftment without significantly increasing morbidity or mortality in patients with SCD.32 A recent study reported that increasing the TBI dose from 200 cGy to 400 cGy reduced graft failure while maintaining the safety of NMA conditioning for haploidentical HCT.33
Primary immunodeficiency diseases
Severe combined immune deficiency (SCID) is a group of rare disorders that occur as the result of mutations in 1 of more than a dozen known genes that impede the development or function of the immune system. Patients with SCID and the complete absence of T-cell immunity allows allogeneic HCT to be performed without chemotherapy (in select cases) without a high risk of graft failure.34 One exception is adenosine deaminase deficiency in which enzyme replacement by exogenous infusion or in the form of HCT can restore host immune function. In non-SCID primary immunodeficiency diseases, some type of pretransplant conditioning is always required to achieve sufficient allo-engraftment. Conditioning regimens in this setting often also contain serotherapies such as alemtuzumab or ATG to deplete host graft rejecting cells. Treosulfan and fludarabine can achieve adequate levels of mixed chimerism in children with primary immunodeficiency diseases, including chronic granulomatous disease and Wiskott-Aldrich sydnrome.35-37 Treosulfan-based regimens are considered less toxic than busulfan-based regimens because of a lower rate of veno-occlusive disease and long-term toxicities including infertility.38,39 Treosulfan may have a further advantage over busulfan in young children because it does not cross the blood–brain barrier and may therefore have less neurotoxicity.40
The timing of HCT and an aggressive control of hyperinflammation are critical for a good outcome in patients with immune dysregulation diseases, such as primary hemophagocytic lymphohistiocytosis (HLH). Alemtuzumab is efficient at depleting T cells and also CD52-expressing antigen-presenting cells, which may effectively treat residual/smoldering HLH at the time of transplantation. When given with fludarabine and melphalan, proximal (close to graft infusion time) dosing of alemtuzumab is shown to be associated with a high incidence of mixed chimerism, whereas distal (more distant from graft infusion time) dosing is associated with less mixed chimerism but more acute GVHD.41 In contrast, intermediate dose alemtuzumab (1 mg/kg divided over days −14 to −10) decreases the risk of mixed chimerism and carries a minimal risk of upfront acute GVHD.41,42 Several reports have documented high rates of mixed chimerism and graft failure in transplanted patients with HLH, especially when a RIC is used.43,44 Innovative treatments are thus needed to improve disease control before HCT. Emapalumab, a neutralizing high affinity mAb that binds to receptor-bound and free interferon-γ present in elevated levels, was US Food and Drug Administration approved for pediatric and adult primary patients with HLH and refractory, recurrent, progressive, or intolerant to conventional therapy.45 Most emapalumab-treated patients proceeded to allogeneic HCT and had outcomes that compared favorably to other MAC or RIC regimens.46
Inherited metabolic disorders
Early allogeneic HCT for inherited metabolic disorders primarily used MAC with busulfan and cyclophosphamide and was associated with significant regimen-related toxicity.47 Multiple adjustments to busulfan administration (decreased dose, switching from oral to intravenous formulations, pharmacokinetic-directed dosing) and substituting cyclophosphamide with fludarabine to enhance immune suppression have resulted in similar efficacy with reduced toxicity.48,49 A less “intensive” regimen of treosulfan or melphalan combined with fludarabine is less toxic, but may be associated with rejection or low-level chimerism requiring the need for retransplantation.50
Novel antibody-based conditioning
Targeted agents for conditioning hold promise in being able to enhance intensity of conditioning without adding significant off-target toxicity. Preclinical studies have identified CD117 and CD45 as targets suitable for nongenotoxic antibody-mediated depletion of host hematopoietic cells.51,52 CD117 is expressed on HSPCs, early thymocytes, mast cells, and some types of innate lymphoid, digestive tract cells, and tumors, including acute myeloid leukemia and melanoma. This restricted expression pattern is ideally suited for myeloablation. Treatment of mice with anti-CD117 (c-kit) mAbs depletes hematopoietic stem cells (HSCs) with hematopoiesis returning within 2 weeks.53 To achieve >99% elimination of host HSCs and vigorous hematopoietic recovery in immune competent mice, anti-CD47 mAb (blocks the “don't eat me” signal) needed to be added.52 Nonhuman primate studies support the safety and efficacy of anti-CD117 mAb for HSC depletion.54 Early data from a phase 1 dose escalation trial (NCT02963064) showed long-term myeloid chimerism and nascent B- and T-lymphocyte generation without toxicities providing proof of concept for safely replacing and/or augmenting MAC or NMA conditioning.55
Anti-CD45 agents including “naked” unconjugated, radio-labeled, and toxin-conjugated mAbs have a broad hematopoietic expression range and are advantageous for combined HSC and immune cell depletion. Radioisotope-labeled anti-CD45 mAbs are in advanced testing in adults with myeloid disorders (NCT02665065), and second-generation agents continue to be developed.56 In contrast to unconjugated or radioisotope anti-CD45 antibody, CD45 antibody-drug conjugates (ADCs) are rapidly cleared, avoiding the potential for depleting donor graft hematopoietic and immune cells or requiring an extended period of myelosuppression if more distal administration is required. CD45 ADCs have shown promise in preclinical models of autologous HCT, evidenced by their ability to efficiently deplete HSCs and immune cells in the periphery, thereby enabling donor HSC engraftment and hematopoietic recovery.57 For congenital immunodeficiencies, conditioning with lytic anti-CD45 mAbs, alemtuzumab, fludarabine, and low-dose cyclophosphamide was well tolerated and high-level donor chimerism was achieved.58 In murine and nonhuman primate studies, a single pretransplant dose of antimurine CD45 ADC as monotherapy permitted high-level allo-engraftment of fully MHC and minor histocompatibility antigen-mismatched donor cells.59,60 Human anti-CD45 ADCs have been generated for future trial consideration. Whether pretransplant CD45 ADCs will prove superior in the clinic is unknown and will await such clinical trials in patients with HCT.
Graft sources
BM, peripheral blood stem cells (PBSCs), and umbilical cord blood (UCB) have all been successfully used graft sources for allogeneic HCT in patients with NMD.3,61-63 BM has traditionally been the standard graft source for NMD, given its significantly lower rates of chronic GVHD and associated mortality relative to PBSCs. However, in addition to its more laborious harvest procedure, BM can be associated with low total nucleated cell (TNC) counts, resulting in slow count recovery and increased rates of graft failure.64 Although not always feasible, higher goal TNC counts for the NMD population may be of benefit. PBSCs are the most frequently used graft source in HCT for malignancy because of ease of collection and robust CD34+ cell counts. PBSCs have historically been avoided in NMD given higher rates of chronic GVHD with traditional GVHD prophylaxis, in part because of higher T-cell content.65 Given the higher CD34+ cell counts, PBSCs may be the preferred graft source if using methods of cellular manipulation. UCB has the advantages of less strict HLA-matching requirements and reduced rates of GVHD because of lower T-cell content. However, its use has been limited by low hematopoietic cell content in a given UCB unit, resulting in impaired engraftment.66,67 Slow immune reconstitution and higher risk for infection have been traditionally associated with UCB transplantation, although these events are strongly influenced by the concurrent use of ATG.68 In addition to the previously mentioned considerations, the inherited nature of certain NMDs may require screening of familial donors for carrier states, leaving unrelated donor sources (UCB or other) as the most feasible option. Ongoing advances in allogeneic HCT practice suggest that new strategies via graft manipulation may address these limitations, mainly by (1) limiting GVHD (discussed later) and (2) improving hematopoietic recovery.
Aside from GVHD, poor or slow hematopoietic recovery is a limitation of HCT in NMDs, especially with UCB.69 To overcome the delays in engraftment, Eurocord recommends a higher cell dose (>5 × 107 TNC/kg) for patients with NMD, including use of double UCB if this dose cannot be achieved with a single unit.70 Furthermore, ex vivo UCB expansion approaches have been developed and evaluated clinically.71 Notably, ex vivo expansion of single UCB units with nicotinamide significantly enhances CD34+ cell counts and shortens time to neutrophil and platelet engraftment in the setting of myeloablative HCT for hematologic malignancies.72,73 This approach also resulted in rapid donor cell engraftment and improvement in disease manifestation in a phase 1/2 study enrolling pediatric SCD.74 In other studies, ex vivo expansion of UCB with an arylhydrocarbon receptor antagonist resulted in a significant increase in CD34+ cells, along with shortened time to neutrophil and platelet engraftment compared with unmanipulated grafts.75 Novel approaches to graft manipulation hold promise in enhancing clinical outcomes and warrant further investigation in the setting of NMDs.
GVHD prevention
Pharmacological approaches
Pharmacological regimens, which are commonly used for GVHD prophylaxis, have traditionally revolved around calcineurin inhibitors (CNIs) paired with another agent such as methotrexate, sirolimus, or mycophenolate. CNI-based regimens using either BM or PBSCs clearly result in unacceptable rates of acute and chronic GVHD64 when considering patients with NMD and also have the drawback of prolonged courses of immunosuppressive medications. Historical methods to augment CNI-based regimens have included the addition of polyclonal ATG products, which appears to decrease the incidence of chronic GVHD, albeit at the expense of increased graft rejection and opportunistic infection.76,77 Nevertheless, ATG products remain an important standard component of many platforms for NMD HCT involving fully matched donors. Current trials in malignant disease continue to investigate additions of novel agents to CNI-based platforms including agents targeting CD24,78 CD26,79 α4β7 integrin,80 Janus associated kinase-1 (NCT03320642), and T-cell costimulation.81 Results of these trials will dictate if the addition of any of these novel agents can decrease the risk of GVHD enough to be acceptable for use in patients with NMD.
The emergence of PTCy-based GVHD prophylaxis regimens has been a major advance in transplantation, especially when using haploidentical or mismatched unrelated donor transplants.82,83 Use of such a platform with haploidentical donors for NMDs has been described in patients with SCD,84 other hemoglobinopathies,85 and aplastic anemia.86-88 Analyses demonstrate that BM continues to be associated with lower rates of GVHD than PBSCs in this setting as well.89 The downsides to PTCy-based regimens include the frequent use of TBI during conditioning, exposure to high-dose alkylating agents, delayed engraftment compared with conventional CNI-based regimens and concerns regarding CD8 T-cell dysfunction and kinetics of immune reconstitution.90 In addition, there are some questions if PTCy is as effective in the very young pediatric population.91 It remains to be seen if PTCy-based regimens will become a standard platform upon which to build for patients with NMD and if further improvements in GVHD prevention or immune reconstitution can be made. PTCy-based regimens may emerge as an ideal platform for older patients with NMDs given the well documented success with NMA conditioning.92
Ex vivo manipulation
Ex vivo manipulation of donor grafts involves either negative or positive selection of specific cellular subsets in order to decrease the risk of GVHD, commonly referred to as T-cell depletion (TCD). The central question is whether there is sufficient knowledge and technology to modify grafts to achieve lower levels of GVHD, yet to maintain engraftment with minimal rates of opportunistic infection. Inherent to removing donor T cells from the graft is an increased incidence of graft rejection; thus, the vast majority of platforms using ex vivo TCD have involved myeloablative conditioning.
Ex vivo TCD by CD34+ positive selection has been used to deplete allo-reactive T lymphocytes and other immune effector cells from PBSC grafts.93 This technique has resulted in successful engraftment and low rates of acute and chronic GVHD in patients with hematologic malignancies, although it has not been widely investigated for NMD.94-96 Presently, the most widely used CD34+ cell selection platform is the ClinicMACS system (Miltenyi Biotech, Bergisch-Gladbach, Germany) that is Food and Drug Administration approved for patients with acute myeloid leukemia in first remission receiving HCT from a matched sibling donor.97 For patients with NMDs, CD34+ selected TCD has only been shown to be successful for patients with matched sibling donors or in children with SCID. Fortunately, techniques have evolved from CD34+ positive selection that depletes all other cells to more sophisticated manipulation including targeting for depletion CD3+/CD19+,98,99 TCRαβ+,100 or CD45RA+ subsets. This approach is thought to better maintain natural killer cells as well as other T-cell subsets (γδ T cells in the case of αβ depletion) with lower GVHD potential to allow better engraftment and potentially improve immune reconstitution after HCT. Multiple clinical trials in pediatric subjects with NMDs, including hemoglobinopathies, primary immunodeficiencies, and FA, have now demonstrated that αβ T-cell graft depletion results in low rates of both acute and chronic GVHD, compared with what would be expected with T-cell replete HCT, in the setting of haploidentical, mismatched related, and matched unrelated donor transplants.101-104
Efforts to improve outcomes after TCD HCT have also involved the infusion of specific cellular products after HCT. Preliminary data have been reported regarding a pilot study in children with NMDs who received infusions of donor T cells transduced with the inducible suicide gene Caspase 9 (iC9) that rapidly eliminates infused donor T cells should GVHD arise.105 This system is based on a small molecule dimerizing drug, AP1903 (Rimiducid), which homodimerizes a FKBP12 analog that contains modified iC9. Once dimerized, activation of iC9 results in eradication of 99% of iC9-expressing T cells within 2 hours of a single dose of AP1903 and controls GvHD within 24 to 48 hours.106 There is a 1000-fold lower affinity of AP1903 to the wild-type FKBP12 because of a single amino acid substitution, thereby providing selectivity for transduced T cells.
Infusion of ex vivo expanded or freshly isolated, highly purified regulatory T cells (Tregs) have been shown to decrease GVHD after UCB or haploidentical HCT.107,108 Recently, a novel technology (ORCA Bio) that rapidly sorts and purifies cellular subsets to single-cell precision was shown to have a compellingly low incidence of significant GVHD in adult patients with acute leukemia given CD34+ selected HCT with purified Tregs, then followed 2 days later by donor-derived conventional T-cell infusion.109,110 The potential applications exist for customized grafts based on factors such as underlying disease as well as specific host-donor combinations.
Current and future trials will determine if progress can be made in GVHD prevention regimens for patients with NMD undergoing HCT. It remains to be seen if any of the novel agents added to standard CNI-based regimens will emerge as a new standard of care. Although PTCy appears to prevent GVHD better than traditional CNI regimens, there are clear drawbacks to PTCy as discussed previously, despite being attractive given its success with RIC. Ex vivo graft manipulation holds much promise and has evolved in recent years. However, expertise, facilities, logistics, and costs of ex vivo manipulations as well as toxicity associated with MAC have limited the widespread adoption of these techniques much beyond academic centers. As discussed, targeted conditioning agents with less nonspecific toxicity may be able to overcome this obstacle in the future. Ultimately, disorders with single gene mutations will likely be better served with genetically modified autologous graft strategies (covered in an accompanying article in this Blood review series), which will eliminate any risk of GVHD. Although the observations of single lineage or mixed chimerism can effectively treat specific diseases, it seems unlikely that any platform will be able to reliably produce such a sustainable stable state in different individual patients. Graft engineering technology with the ability to specifically customize graft components with single-cell precision has great potential to ultimately engender successful engraftment and minimize risks of GVHD and opportunistic infection, especially for patients with NMDs, and results of early trials with such technology are eagerly awaited.
Additional considerations
Immune tolerance mechanisms
In an individual patient, a threshold level of donor anti-host alloreactive T cells must be reached to cause GVHD. Immune modulatory mechanisms can elevate this threshold by counterbalancing the efficiency of donor T cells to mediate tissue destruction and GVHD. For illustration, we will discuss mechanisms of PTCy in light of the increasingly widespread use of the GVHD prevention approach. Reduced GVHD rates seen in patients receiving PTCy have been attributed to CD8 T-cell dysfunction that provides a more permissive environment for thymic Treg preservation and peripheral Treg generation.111,112 The net result of a diminished donor anti-host T-cell burden coupled with an augmentation in peripheral regulatory mechanisms is a shift in the balance of donor T cells from a dominant state of alloreactivity to immune regulation and tolerance. Host hematopoietic cells do not appear to contribute to lower GVHD rates because there is no mixed chimerism in PTCy-treated aplastic anemia or SCD.33,87 Moreover, hematopoietic cells in these and other NMDs tend to reach >95% engraftment or are rejected.113 Inclusion of pretransplant ATG and TBI doses of 400 cGy serve to minimize rejection rates.33 although specific mechanisms associated with lower GVHD rates seen with other approaches may have distinct tolerogenic mechanisms, raising the threshold for donor anti-host alloreactive serves is a common denominator.
Stable mixed chimerism
Many patients with NMD do not require complete donor chimerism to correct the disease phenotype. Stable mixed chimerism has a well-documented ameliorated effect in thalassemia and SCD. In SCD, only 20% donor myeloid chimerism is required to reverse the sickle phenotype.114,115 Yet, the greatest concern is that stable donor chimerism will not occur because of transfusion-based allosensitization coupled with a robustly hyperplastic BM.116,117 Serial testing can detect decreases in donor chimerism that may herald graft rejection,118 although this must be evaluated carefully in context of mixed chimerism.18 Although a mixed chimerism state can be managed successfully with a prolonged taper of immune suppression, not all NMDs can be managed with this approach. In Hurler syndrome, full donor chimerism contributed to normal leukocyte IDUA enzymes levels after transplant, which was a predictor of superior long-term outcomes.9
Pretransplant therapy or alternative conditioning approaches may enhance donor chimerism after allogeneic HCT. Chimerism data reported in the phase 2/3 trial of emapalumab in HLH are promising119 because complete donor chimerism was observed in all but 1 surviving patient, as opposed to several reports of high rates of mixed and often unstable chimerism in transplanted HLH, especially when RIC regimens are used.43,120 In adult patients with severe congenital anemias, an ongoing NMA allogeneic HCT trial with radiation, alemtuzumab, and sirolimus (NCT00061568) has preliminary results showing that stable, mixed chimerism can be achieved.121
There are no generally accepted, successful interventions for increasing donor-specific engraftment in the setting of mixed chimerism. In certain situations, based upon declining chimerism levels and in consultation with a transplant center with allogeneic HCT expertise in the underlying disorder, donor lymphocyte infusion may be indicated although experience in NMDs has been limited and is associated with a significant risk of GVHD. Repeat allogeneic HCT may be effective in selected patients, particularly those treated later posttransplant after recovering from regimen-related toxicities,122 although results for treatment of neutropenic graft failure are much worse than those with non-neutropenic graft failure.123
Immune-mediated cytopenias
Corticosteroids and/or intravenous immunoglobulin along with supportive therapy (eg, use of granulocyte colony-stimulating factor and blood product transfusions) are the mainstays of treatment of IMCs.16,124 Daratumumab, an anti-CD38 mAb that can eliminate CD38 high plasma cells, is now the preferred upfront therapy in moderate to severe IMCs.125,126 Agents such as rituximab, which target CD20+ B cells, also have shown efficacy in IMCs,127,128 suggesting an important role for B cells. More effective B-cell depletion can be achieved with newer agents such as obinutuzumab, a humanized, type II, anti-CD20 mAb, which acts through antibody-dependent cellular cytotoxicity and leads to direct apoptosis of mature B cells.129 In a different approach, the proteasome inhibitor, bortezomib, has been used to targets T cells and plasma cells, which produce antibodies directed against 1 or more hematopoietic lineages.129 Because the majority of patients develop IMCs while on post-HCT immunosuppression, another important approach is potentially switching the calcineurin inhibitors to sirolimus, which has shown improvement in recurrent and refractory IMCs.130
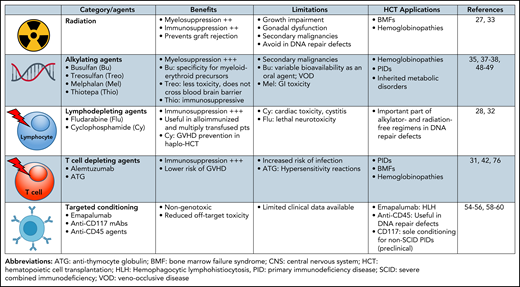
Current and emerging preparative regimens used in allogeneic HCT for nonmalignant disease.
Current and emerging preparative regimens used in allogeneic HCT for nonmalignant disease.
Conclusion
NMDs possess unique challenges as it pertains to the successful implementation of allogeneic HCT. However, the emergence of novel approaches to prevention of graft rejection, GVHD, and IMCs hold promise in overcoming these historical barriers. Because many initial studies are conducted for subjects with hematologic malignancies, carefully planned collaborative clinical trials will need to explore these novel transplant approaches in the NMD population to advance clinical care.
Acknowledgments
The authors thank Paul Orchard, Margaret MacMillan, and Angela Smith for helpful discussions.
Authorship
Contribution: Z.D., M.H., Y.-B.C., and B.R.B. wrote the review.
Conflict-of-interest disclosure: Z.D. receives research support from Incyte Corp. and Regimmune Corp. and has received consulting fees from Syndax Pharmaceuticals Inc. and Omeros Corp. Y.-B.C. has received consulting fees from Incyte, Gamida Cell, Equilium, Celularity, Daiichi, Actinium, and Abbvie. B.R.B. serves on advisory boards for Magenta Therapeutics and BlueRock Theapeutics; receives research funding from BlueRock Therapeutics, Rheos Medicines, Equilibre Pharmaceuticals Corp., and Carisma Therapeutics, Inc; is a cofounder of Tmunity Therapeutics; and receives the following grant support: National Institutes of Health National Institute of Allergy and Infectious Diseases (R37 AI34495) and National Heart, Lung, and Blood Institute (R01 HL147324, R01 HL155114, R01 HL56067). M.H. declares no competing financial intererests.
Correspondence: Zachariah DeFilipp, Massachusetts General Hospital, 55 Fruit St, Zero Emerson Pl, Suite 118, Office 134, Boston, MA 02114; e-mail: zdefilipp@mgh.harvard.edu.