Abstract
The erythroid marrow and circulating red blood cells (RBCs) are the key components of the human erythron. Abnormalities of the erythron that are responsible for anemia can be separated into 3 major categories: erythroid hypoproliferation, ineffective erythropoiesis, and peripheral hemolysis. Ineffective erythropoiesis is characterized by erythropoietin-driven expansion of early-stage erythroid precursors, associated with apoptosis of late-stage precursors. This mechanism is primarily responsible for anemia in inherited disorders like β-thalassemia, inherited sideroblastic anemias, and congenital dyserythropoietic anemias, as well as in acquired conditions like some subtypes of myelodysplastic syndrome (MDS). The inherited anemias that are due to ineffective erythropoiesis are also defined as iron-loading anemias because of the associated parenchymal iron loading caused by the release of erythroid factors that suppress hepcidin production. Novel treatments specifically targeting ineffective erythropoiesis are being developed. Iron restriction through enhancement of hepcidin activity or inhibition of ferroportin function has been shown to reduce ineffective erythropoiesis in murine models of β-thalassemia. Luspatercept is a transforming growth factor-β ligand trap that inhibits SMAD2/3 signaling. Based on preclinical and clinical studies, this compound is now approved for the treatment of anemia in adult patients with β-thalassemia who require regular RBC transfusions. Luspatercept is also approved for the treatment of transfusion-dependent anemia in patients with MDS with ring sideroblasts, most of whom carry a somatic SF3B1 mutation. While the long-term effectiveness and safety of luspatercept need to be evaluated in β-thalassemia and MDS, defining the molecular mechanisms of ineffective erythropoiesis in different disorders might allow the discovery of new effective compounds.
Introduction
The human erythron is the highly specialized tissue that is responsible for oxygen transport.1 Its key components are the erythroid marrow, which is responsible for red cell production (erythropoiesis), and circulating red blood cells (RBCs), which transport oxygen from the lung to every cell in the body (Figure 1).2 In normal individuals, approximately 2.5 × 106 reticulocytes are released into the circulation every second. After spending about 120 days in circulation, senescent RBCs are phagocytized by the reticuloendothelial cells in the spleen, the liver, and the bone marrow itself. Erythropoiesis is primarily regulated by erythropoietin (Epo), which is produced by the oxygen-sensing apparatus in the kidney, circulates in peripheral blood as a true hormone, and exerts its effects by binding to a surface receptor (EPOR) present on erythroid progenitors and precursors in the bone marrow.2
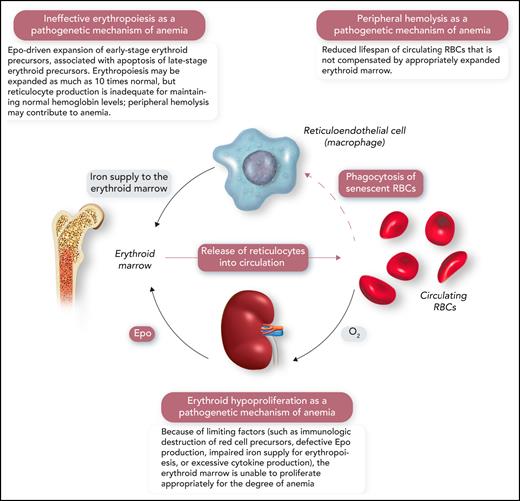
A schematic representation of the human erythron (erythroid marrow plus circulating RBCs), the kidney oxygen-sensing apparatus, and the reticuloendothelial system that phagocytizes senescent RBCs and returns iron to the erythroid marrow. Erythroid hypoproliferation, ineffective erythropoiesis, and peripheral hemolysis are the 3 major categories of a functional classification of anemia. Professional illustration by Somersault18:24.
A schematic representation of the human erythron (erythroid marrow plus circulating RBCs), the kidney oxygen-sensing apparatus, and the reticuloendothelial system that phagocytizes senescent RBCs and returns iron to the erythroid marrow. Erythroid hypoproliferation, ineffective erythropoiesis, and peripheral hemolysis are the 3 major categories of a functional classification of anemia. Professional illustration by Somersault18:24.
From a pathophysiological point of view, anemia may result from 3 distinct abnormalities of the human erythron that are related to the proliferation of erythroid precursors, their maturation, or red cell survival in the circulation (Figure 1).1,3,4 Consequently, 3 major pathogenetic mechanisms of anemia can be defined: erythroid hypoproliferation, ineffective erythropoiesis, and peripheral hemolysis (Figure 1).1 For instance, erythroid precursors are virtually absent in the bone marrow of patients with severe aplastic anemia or pure red cell aplasia, with minimal or no release of reticulocytes into the circulation. In contrast, the number of immature red cells may be as high as 10 times normal in the bone marrow of patients with β-thalassemia intermedia; nonetheless, red cell production is inadequate because of the apoptosis of late-stage erythroblasts and insufficient production of reticulocytes. In patients with severe hemolytic anemia, the number of reticulocytes released into the circulation may be >10 times normal, but the newly formed red cells often survive for only a few days in the circulation, leading to anemia.
This review article focuses on ineffective erythropoiesis as a pathophysiological mechanism of anemia in different disorders and examines novel treatments that target the abnormal maturation of erythroid precursors and can ameliorate anemia in these conditions.
The groundbreaking studies that led to the notion of ineffective erythropoiesis
When senescent red cells are phagocytized by reticuloendothelial cells (macrophages), hemoglobin is broken down into its components, and the catabolism of the protoporphyrin ring of heme leads to the formation of bilirubin.1 In addition to hemoglobin, several heme-containing proteins are found in the human body; therefore, multiple sources of bilirubin are present. The origins of bilirubin in humans were investigated decades ago; these pioneer studies are schematically summarized in Figure 2.5-9
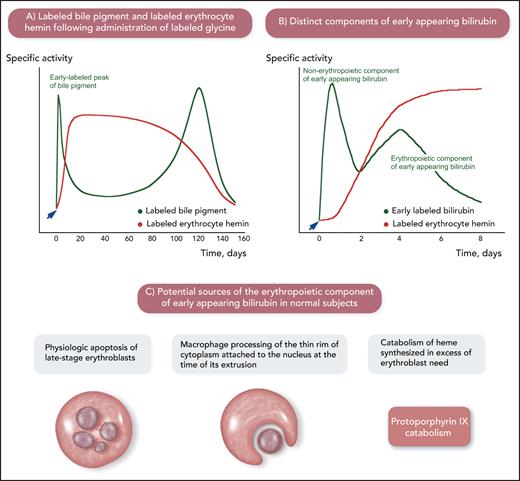
The origins of bilirubin in humans as studied using labeled glycine, a substrate for the biosynthesis of heme. The studies summarized in this figure were conducted decades ago but were of fundamental importance for developing the notion of ineffective erythropoiesis. (A) Physiologic sources of bilirubin. After administration of labeled glycine (arrow), 2 peaks of labeled bile pigment (stercobilin, which derives from bilirubin in the intestine) were detected: one, corresponding to 10% to 20% of total labeled pigment, in the first few days after administration of labeled glycine and the second one at approximately 120 days, at the time of maximal disappearance of labeled hemin. This is a schematic representation of the experiments performed by London et al.5 (B) Components of early appearing bilirubin after administration of labeled glycine (arrow): 2 distinct peaks are visible in normal subjects. The first one after 12 to 24 hours has been related to the catabolism of porphyrins and heme-containing proteins in the liver (nonerythropoietic component of early appearing bilirubin). The second one on days 3 to 5 has been related to the catabolism of heme in bone marrow erythroid precursors (erythropoietic component of early appearing bilirubin). This is a schematic representation of the experiments performed by Yamamoto et al.6 (C) Potential sources of the erythropoietic component of early appearing bilirubin. Apoptosis of late-stage erythroblasts that are not reached by sufficient amounts of Epo and processing of the thin rim of cytoplasm attached to the nucleus at the time of its extrusion can be considered physiologic ineffective erythropoiesis. Professional illustration by Somersault18:24.
The origins of bilirubin in humans as studied using labeled glycine, a substrate for the biosynthesis of heme. The studies summarized in this figure were conducted decades ago but were of fundamental importance for developing the notion of ineffective erythropoiesis. (A) Physiologic sources of bilirubin. After administration of labeled glycine (arrow), 2 peaks of labeled bile pigment (stercobilin, which derives from bilirubin in the intestine) were detected: one, corresponding to 10% to 20% of total labeled pigment, in the first few days after administration of labeled glycine and the second one at approximately 120 days, at the time of maximal disappearance of labeled hemin. This is a schematic representation of the experiments performed by London et al.5 (B) Components of early appearing bilirubin after administration of labeled glycine (arrow): 2 distinct peaks are visible in normal subjects. The first one after 12 to 24 hours has been related to the catabolism of porphyrins and heme-containing proteins in the liver (nonerythropoietic component of early appearing bilirubin). The second one on days 3 to 5 has been related to the catabolism of heme in bone marrow erythroid precursors (erythropoietic component of early appearing bilirubin). This is a schematic representation of the experiments performed by Yamamoto et al.6 (C) Potential sources of the erythropoietic component of early appearing bilirubin. Apoptosis of late-stage erythroblasts that are not reached by sufficient amounts of Epo and processing of the thin rim of cytoplasm attached to the nucleus at the time of its extrusion can be considered physiologic ineffective erythropoiesis. Professional illustration by Somersault18:24.
After administration of labeled glycine (Figure 2A-B), the so-called “early appearing bilirubin” includes 2 components: a nonerythropoietic component and an erythropoietic component.6 The latter coincides with the maximal increment in labeled hemin of circulating red cells and, therefore, has been associated with erythropoiesis.6,9 As illustrated in Figure 2C, apoptosis of a small portion of erythroblasts that are not reached by sufficient amounts of Epo, macrophage processing of the thin rim of cytoplasm around the extruded erythroblast nucleus, and catabolism of heme produced in excess of the erythroblast need for hemoglobin synthesis may all contribute to the erythropoietic component of early appearing bilirubin in normal subjects. A number of studies showed that this component increases considerably in patients with megaloblastic anemia, β-thalassemia, and sideroblastic anemia.6,9 The common denominator of these conditions is the expansion of the erythroid marrow associated with defective red cell production; thus, the increase in early appearing bilirubin in these patients suggests that it derives from the destruction of nonviable erythroblasts in the bone marrow.10 The erythroid marrow is active and expanded but its efficacy in terms of red cell production is impaired, hence the term “ineffective erythropoiesis.”
The notion of ineffective erythropoiesis was further developed based on combined studies of iron kinetics and red cell survival (so-called erythrokinetic studies).11 Ineffective erythropoiesis was shown to be characterized by increased erythron iron turnover associated with reduced RBC utilization.3,12-14 Using mathematical models for the analysis of ferrokinetic data, the percentage of ineffective erythropoiesis was estimated to be on the order of 5% of total erythroid activity in normal individuals.14 This calculation was based on measurements of erythroid marrow iron turnover (total erythropoiesis), red cell iron turnover (effective erythropoiesis), and ineffective red cell iron turnover (ineffective erythropoiesis). Approximately 95% of the iron taken up by erythroid cells in the bone marrow was ultimately detected in circulating RBCs, indicating that ∼95% of red cell production is effective, whereas 5% is ineffective, under normal conditions.
Anemic disorders characterized by ineffective erythropoiesis
Ineffective erythropoiesis is the major pathogenetic mechanism that is responsible for anemia in several inherited and acquired disorders (Table 1).15-18 In these conditions, the bone marrow is characterized by erythroid hyperplasia, whereas the number of circulating reticulocytes is inadequate for the degree of anemia.1 In fact, the reticulocyte production index is typically <2, whereas this parameter is >3 in patients with hemolytic anemia (Table 2).1
Distinguishing between ineffective erythropoiesis and erythroid hypoproliferation as a mechanism of anemia may be problematic in patients with myelodysplastic syndrome (MDS), essentially because no quantitative measurements of erythroid activity and its efficacy are available in a clinical setting. When bone marrow is examined for diagnostic purposes, the myeloid/erythroid (M/E) ratio can be used as an index of erythroid activity.1 The M/E ratio is the ratio of myeloid precursors to erythroid precursors and varies from 2:1 to 4:1 in normal individuals. In patients with ineffective erythropoiesis, erythroblasts are predominant in the bone marrow, and the M/E ratio is <1:1; in contrast, it is >1:1 in patients with erythroid hypoproliferation. Patients with ineffective erythropoiesis have normal to increased levels of unconjugated bilirubin; the overproduction derives from the catabolism of the hemoglobin of apoptotic erythroid precursors in bone marrow macrophages. These patients often have evidence of parenchymal iron overload, which derives from suppression of hepcidin production, as discussed under “The impact of ineffective erythropoiesis on iron metabolism: the concept of iron loading anemia.” Typically, they have elevated levels of circulating soluble transferrin receptor, despite normal or increased body iron stores, which reflect the increased number of transferrin receptors associated with expansion of the erythroid marrow.19,20
The impact of ineffective erythropoiesis on iron metabolism: the concept of iron loading anemia
Body iron stores represent the primary regulator of iron balance in humans.21 The existence of an erythroid regulator of iron balance was proposed by Finch based on experimental and clinical observations.22 This regulator had to be in a tissue highly sensitive to iron supply; the erythroid marrow meets this qualification because it uses >80% of plasma iron. By comparing patients with anemia due to ineffective erythropoiesis and patients with hemolytic anemia, intestinal iron absorption was found to be significantly higher in the former subjects, pointing to a crucial role for intramedullary erythroid cell death.23
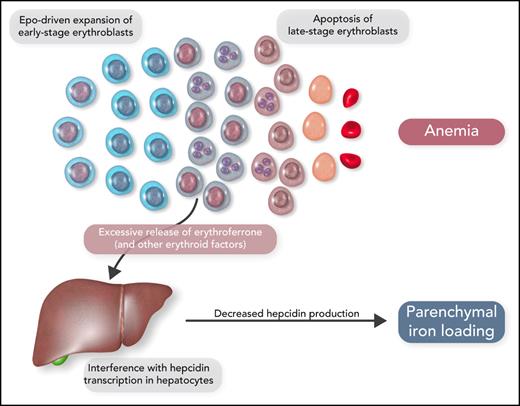
It has now become clear that the expanded erythroid marrow of patients with ineffective erythropoiesis involves an excessive release of factors that may have an impact on iron metabolism (Figure 3). Growth differentiation factor 15 levels were found to be increased in β-thalassemia and congenital dyserythropoietic anemia type I.24,25 Erythroferrone was then identified as a new hormone that is produced by erythroblasts and inhibits the synthesis of hepcidin in the liver26; notably, erythroid overproduction of erythroferrone causes iron overload in mice.27 In β-thalassemia, the release of increased amounts of erythroferrone by the expanded erythroid marrow suppresses hepcidin secretion, thus increasing iron absorption from the gut and iron release by reticuloendothelial cells and leading, in turn, to parenchymal iron overload, even in the absence of RBC transfusion.28
Schematic representation of the molecular mechanisms that lead to parenchymal iron loading in inherited anemias associated with ineffective erythropoiesis, the so-called “iron-loading anemias.” The most representative iron-loading anemias are nontransfusion-dependent β-thalassemia, inherited sideroblastic anemias, and congenital dyserythropoietic anemias. Professional illustration by Somersault18:24.
Schematic representation of the molecular mechanisms that lead to parenchymal iron loading in inherited anemias associated with ineffective erythropoiesis, the so-called “iron-loading anemias.” The most representative iron-loading anemias are nontransfusion-dependent β-thalassemia, inherited sideroblastic anemias, and congenital dyserythropoietic anemias. Professional illustration by Somersault18:24.
Because of the strong association between ineffective erythropoiesis and parenchymal iron loading due to increased iron absorption, the inherited disorders of Table 1 have been defined as “iron-loading anemias.”21 The most representative conditions are nontransfusion-dependent β-thalassemia, inherited sideroblastic anemias, and congenital dyserythropoietic anemias.17,20,29 Some patients with sideroblastic anemia or congenital dyserythropoietic anemia have only mild anemia or even borderline hemoglobin levels; the most relevant clinical manifestations are those related to parenchymal iron overload.30
An accurate evaluation of body iron status in patients with ineffective erythropoiesis requires, at minimum, the assessment of serum iron, transferrin saturation, and serum ferritin. Magnetic resonance imaging is used for the quantification of iron concentration in target organs in thalassemic patients.31 It remains to be established whether hepcidin measurement is clinically useful in these patients; the same holds true for serum erythroferrone.32-34
Ineffective erythropoiesis in β-thalassemia
As underscored by Modell in 1974,35 “ineffective erythropoiesis is the outstanding feature of thalassemia major.” In untreated patients with transfusion-dependent β-thalassemia or nontransfusion-dependent β-thalassemia, the erythroid cells expand to occupy all of the available space in the bone marrow. When the expansion is significant, erythroid cells can accumulate outside of the bone marrow, generating extramedullary hematopoiesis.31 In patients with thalassemia syndromes, ineffective erythropoiesis is typically found in those with β-thalassemia or β-thalassemia/hemoglobin E disease, whereas the anemia of patients with hemoglobin H disease is mainly due to peripheral hemolysis.3,12,36
The role of excess α-globin chains
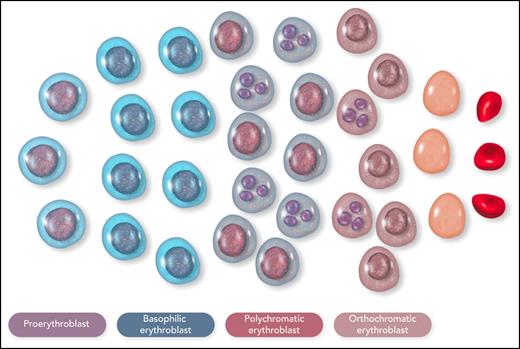
β-Thalassemia is characterized by defective production of β-globin chains with the accumulation of excess unstable α-globin chains in erythroid cells.31 In the bone marrow of a patient with nontransfusion-dependent β-thalassemia, most of the erythroid cells are proerythroblasts or basophilic erythroblasts, with an arrest of maturation in the polychromatic erythroblasts that is associated with cellular precipitates of insoluble α-globin chains (Figure 4).37,38 The intramedullary cell death, or apoptosis, of late-stage erythroblasts represents the pathophysiological mechanism that leads to ineffective erythropoiesis.39-41 A hemolytic component of variable degree, due to the presence of insoluble α-globin chains in circulating red cells, contributes to anemia. The overproduction of Epo dictated by the kidney oxygen-sensing apparatus expands early erythropoiesis by preventing apoptosis of erythroid progenitor cells and early-stage erythroblasts,42,43 but it is unable to prevent apoptosis of polychromatic erythroblasts with α chain precipitates.40 In addition, very high rates of erythroid proliferation might generate an increased number of apoptotic cells41 or impair erythroid maturation,44 thus establishing a vicious cycle.
Schematic representation of the cellular composition of ineffective erythropoiesis in β-thalassemia. Most of the erythroid cells are proerythroblasts or basophilic erythroblasts, with an arrest of maturation in the polychromatic erythroblasts; many polychromatic and orthochromatic erythroblasts, exemplified here as cells with a fragmented nucleus, undergo apoptosis. This figure illustrates the notion that ineffective erythropoiesis is characterized by Epo-driven expansion of early-stage erythroid precursors, associated with apoptosis of late-stage erythroid precursors. Erythroid cells were reproduced from Servier Medical Art (smart.servier.com). Professional illustration by Somersault18:24.
Schematic representation of the cellular composition of ineffective erythropoiesis in β-thalassemia. Most of the erythroid cells are proerythroblasts or basophilic erythroblasts, with an arrest of maturation in the polychromatic erythroblasts; many polychromatic and orthochromatic erythroblasts, exemplified here as cells with a fragmented nucleus, undergo apoptosis. This figure illustrates the notion that ineffective erythropoiesis is characterized by Epo-driven expansion of early-stage erythroid precursors, associated with apoptosis of late-stage erythroid precursors. Erythroid cells were reproduced from Servier Medical Art (smart.servier.com). Professional illustration by Somersault18:24.
Different molecular mechanisms are likely responsible for the excessive apoptosis of polychromatic erythroblasts. In normal erythropoiesis, the molecular chaperone α-hemoglobin–stabilizing protein is sufficient to prevent the deleterious effects of free α-globin precipitation.45 In contrast, it is insufficient to control the excess unstable α-globin chains in β-thalassemia, which leads to increased reactive oxygen species and oxidative damage in erythroid cells.46 The chaperone heat shock protein 70 plays a role in human erythropoiesis, translocating into the nucleus of maturating erythroblasts and, thus, preventing caspase-3–mediated cleavage of the transcription factor GATA-1.47 In thalassemic erythroblasts, heat shock protein 70 binds to free α-globin chains and remains sequestered in the cytoplasm. Therefore, it is unable to reach the nucleus and protect GATA-148; in turn, reduced levels of GATA-1 in the nucleus cause maturation arrest and apoptosis of polychromatic erythroblasts. Recent studies identified molecules that may play a compensatory role in conditions with ineffective erythropoiesis: in β-thalassemic mice, pleckstrin-2 was found to prevent apoptosis and enhance the enucleation of immature red cells.49
Treatment of ineffective erythropoiesis in β-thalassemia
Regular RBC transfusion therapy represented the first effective treatment for ineffective erythropoiesis in patients with β-thalassemia.50 Increasing hemoglobin levels through RBC transfusions suppresses the overproduction of Epo and the related expansion of erythropoiesis. However, the major adverse event is transfusion iron overload with its detrimental consequences; this led to the use of iron chelation therapy.51 Regular RBC blood transfusions and iron chelation with deferoxamine markedly changed the prognosis of β-thalassemia major, significantly improving the survival of these patients.52
In recent years, several studies aimed at specifically targeting ineffective erythropoiesis in β-thalassemia have been conducted. In animal models, administration of hepcidin, hepcidin agonists, molecules that increase hepcidin expression, or a ferroportin inhibitor was shown to variably ameliorate ineffective erythropoiesis and iron overload.53-59 The molecular mechanisms by which iron restriction ameliorates ineffective erythropoiesis remain largely unclear, but a few clinical trials based on this therapeutic strategy are ongoing in patients with nontransfusion-dependent β-thalassemia. One of these studies evaluates the efficacy and safety of an antisense oligonucleotide that downregulates TMPRSS6 and stimulates hepcidin production (TMPRSS6-LRx; ClinicalTrials.gov Identifier: NCT04059406). Another study evaluates the efficacy and safety of an oral ferroportin inhibitor that reduces iron release from reticuloendothelial cells (VIT-2763; ClinicalTrials.gov Identifier: NCT04364269).
The transforming growth factor-β (TGF-β) family includes several cytokines, such as activins and bone morphogenetic proteins, that play different roles in human hematopoiesis.60 All ligands of this family signal through cell surface receptors, and ligand/receptor binding activates the SMAD signaling circuitry. Molecules that bind ligands preventing ligand/receptor interaction and inhibiting signaling are known as ligand traps; 2 of these molecules are luspatercept and sotatercept. This latter was originally developed to increase bone mineral density in postmenopausal women, but a clinical trial unexpectedly showed dose-dependent stable increases in hemoglobin, hematocrit, and red blood cell counts.61 This observation led to studies aimed at evaluating the efficacy of TGF-β ligand traps in the treatment of anemia, and a mouse version of sotatercept was found to reverse ineffective erythropoiesis and ameliorate anemia in β-thalassemia mice.62 This effect was confirmed in a clinical trial on the use of sotatercept in the treatment of patients with β-thalassemia.63 The initial study in β-thalassemic mice suggested that the effect of sotatercept was related to inhibition of GDF11.62 However, a subsequent study showed that GDF11 is not the target of TGF-β ligand traps and that decreasing GDF11 in immature red cells has no effect on anemia.64 Recent observations in murine β-thalassemia suggest that Smad2/3 pathway overactivation contributes to ineffective erythropoiesis by reducing nuclear levels of GATA-1 in erythroid precursors.65 Luspatercept would sequester Smad2/3 pathway ligands, prevent overactivation of this pathway, and increase the nuclear availability of GATA-1, ultimately improving erythroid maturation.65 Although these observations are interesting, the mechanisms of action of TGF-β ligand traps on ineffective erythropoiesis remain largely to be elucidated.
Following the above-mentioned studies on the therapeutic use of TGF-β ligand traps, luspatercept was developed for the treatment of β-thalassemia. An open-label nonrandomized uncontrolled study showed that luspatercept could improve hemoglobin levels and RBC transfusion requirements in these patients.66 A randomized double-blind phase 3 trial (BELIEVE trial) was then conducted in adults with transfusion-dependent β-thalassemia; 224 patients were assigned to the luspatercept group, and 112 patients were assigned to the placebo group.67 At any 12-week interval during the study, the proportion of patients who had a reduction in the RBC transfusion burden ≥50% was greater in the luspatercept group (40.2%) than in the placebo group (6.3%). Treatment was associated with some adverse events, such as bone pain and arthralgia, and, of note, with a higher incidence of thromboembolic events. While a 5-year open-label extension-phase study to evaluate long-term safety is underway (ClinicalTrials.gov Identifier: NCT04064060), the BELIEVE trial shows that luspatercept can reduce transfusion requirements in patients with transfusion-dependent β-thalassemia. Luspatercept is approved for the treatment of anemia in adult patients with β-thalassemia who require regular red cell transfusions. A clinical study has been planned to determine the efficacy and safety of luspatercept in adults with nontransfusion-dependent β-thalassemia (BEYOND trial; ClinicalTrials.gov Identifier: NCT03342404).
Mitapivat is an allosteric activator of pyruvate kinase.68 In a controlled phase 2 study of patients with pyruvate kinase deficiency, oral administration of mitapivat resulted in pyruvate kinase activation and sustained clinically significant hemoglobin increases.69 In a mouse model of β-thalassemia, mitapivat was shown to reduce hemolysis and improve anemia.70 Clinical trials aimed at evaluating the efficacy and safety of mitapivat in thalassemic patients have been registered at ClinicalTrials.gov.
Ineffective erythropoiesis in inherited sideroblastic anemias
The best characterized nonsyndromic inherited sideroblastic anemias are X-linked sideroblastic anemia, associated with ALAS2 mutations, and autosomal-recessive sideroblastic anemia, associated with SLC25A38 mutations (Table 1).15
Males with X-linked sideroblastic anemia may present in the first 2 decades of life with symptoms of anemia or later with manifestations of anemia or parenchymal iron overload.30 Most patients are responsive to pyridoxine treatment, with a significant reduction in ineffective erythropoiesis and a variable degree anemia amelioration. Under pyridoxine supplementation, patients with parenchymal iron overload can safely undergo mild phlebotomy programs to normalize body iron status.
The vast majority of patients with autosomal-recessive sideroblastic anemia associated with SLC25A38 mutations present at birth or infancy with severe microcytic anemia that requires chronic transfusion and iron chelation therapy.71 Allogeneic stem cell transplantation represents the only curative therapy.
Ineffective erythropoiesis in congenital dyserythropoietic anemias
A comprehensive review of congenital dyserythropoietic anemias (CDAs) was published recently in this journal.18 Although the major mechanism of anemia in patients with CDAs is ineffective erythropoiesis, a variable degree of peripheral hemolysis is often present, especially in CDA type II.72 Most patients have evidence of parenchymal iron overload in middle age, and the degree of iron overload is related to the expansion of erythropoiesis.20,29 In a cellular model of CDA type II, RAP-011, an ortholog of sotatercept, was found to rescue the disease phenotype by inhibiting the SMAD2-3 pathway.73 Improving the efficiency of erythropoiesis in patients with CDA type II might ameliorate anemia, as well as prevent parenchymal iron loading; therefore, ad hoc clinical trials are needed.
Ineffective erythropoiesis in MDSs
MDSs are myeloid neoplasms that are characterized by morphologic dysplasia, variable degrees of ineffective hematopoiesis, and peripheral blood cytopenia.74 They originate from the growth and expansion of a somatically mutated clone of hematopoietic cells and are highly heterogeneous because several mutation-driver genes may be responsible for myelodysplastic hematopoiesis.74
The notion that hematopoiesis is ineffective in MDS was initially based on the observation that, although the bone marrow is hypercellular, these patients have variable combinations of cytopenia, most commonly anemia. A large body of studies indicated that, in initial phases of MDS, increased apoptosis is associated with ineffective hematopoiesis and cytopenia; in contrast, in advanced phases of the disease, impaired differentiation of mutant hematopoietic cells represents the mechanism that leads to the accumulation of progenitors and, ultimately, blasts, resulting in leukemic transformation.75
Quantitative studies of erythroid activity showed that different mechanisms are responsible for anemia in patients with MDS.14,76 Ineffective erythropoiesis was typically found in patients with refractory anemia with ring sideroblasts, which is currently defined as MDS with ring sideroblasts (MDS-RS).14 Conversely, erythroid hypoproliferation was found to be responsible for anemia in the remaining patients, primarily those with MDS with excess blasts.76 Subsequent studies established a relationship between apoptosis of erythroid precursors and ineffective erythropoiesis in MDS-RS.77,78 Within these patients, the SF3B1 mutation identifies those with high degrees of ineffective hematopoiesis resulting in low hepcidin levels and a propensity for parenchymal iron loading.79,80
SF3B1-mutant MDS
Within patients with MDS, those with MDS-RS have relatively indolent disease.81,82 The vast majority of these patients carry a driver somatic mutation in the spliceosome gene SF3B1.83-86 In this condition, hematopoietic cells are heterozygous for the SF3B1 mutation, so that their cytoplasm contains approximately equal proportions of normal and mutated SF3B1 splicing factors. Spliceosomes with mutant SF3B1 splicing factor preferentially use cryptic 3' splice sites, which leads to nonsense–mediated decay of multiple misspliced transcripts or generation of in-frame isoforms.87 Within downregulated genes are ABCB7 and TMEM14C, whose coordinated haploinsufficiency plays a crucial role in ring sideroblast formation.88 In addition, downregulation of SEPTIN2 was shown to contribute to defective erythroid maturation.89
Within patients with lower-risk MDS, the plasma concentration of erythroferrone was found to be higher in those with SF3B1-mutant MDS, whereas plasma hepcidin concentration was lower.90 Of note, mutant SF3B1-related missplicing resulted in the production of a variant erythroferrone molecule that maintained the capacity to suppress hepcidin transcript.90 A recent study confirmed that erythroferrone is overexpressed in CD71+ erythroid cells of patients with MDS, particularly those with an SF3B1 mutation.91
Most patients with SF3B1-mutant MDS have a lower-risk disease, and the main objective of treatment is to ameliorate anemia and improve the quality of life.74 Erythropoiesis-stimulating agents (ESAs) represent the first therapeutic option in anemic patients with lower-risk MDS. The most reliable predictor of response is a serum Epo level <200 mU/mL, and the median duration of response is 1 to 2 years.92,93 The fact that a relatively low Epo level predicts response to ESA is important for understanding the mechanism of action of recombinant Epo. Serum Epo levels are regulated by the rate of renal production, as well as by the rate of utilization by erythroid cells.94 In patients with expanded, but ineffective, erythropoiesis, high rates of Epo utilization are associated with lower serum levels.94 Administration of recombinant human Epo can increase the erythroid hormone levels in the bone marrow, reducing the apoptosis of immature red cells.
In a mouse model of MDS, RAP-536, a murine version of luspatercept, was found to mitigate ineffective erythropoiesis and ameliorate anemia.95 Based on this and other observations, a phase 2 open-label dose-finding study was conducted on the use of luspatercept in anemic patients with lower-risk MDS.96 Approximately two thirds of patients receiving higher doses of luspatercept showed amelioration of anemia; luspatercept treatment was particularly effective in those who had ring sideroblasts, an SF3B1 mutation, or both.96 This led to the design of a double-blind placebo-controlled phase 3 trial in which patients with lower-risk MDS-RS, who had been receiving regular RBC transfusions and were refractory to ESAs, were assigned to receive luspatercept or placebo that was administered subcutaneously every 3 weeks.97 Of the 222 patients enrolled, 202 (91%) carried a somatic mutation in SF3B1. Overall, 38% of patients in the luspatercept group reached transfusion independence for ≥8 weeks compared with 13% in the placebo group.97 Luspatercept has been approved for the treatment of transfusion-dependent anemia in lower-risk patients with MDS-RS.
Conclusions
Ineffective erythropoiesis was defined decades ago, but the molecular mechanisms that lead to the apoptosis of abnormal late-stage erythroblasts remain poorly elucidated. Luspatercept and sotatercept have been identified as compounds that are capable of improving red cell production almost accidentally, and their mechanisms of action are not fully understood. Recent findings indicate that terminal erythroid maturation is controlled largely at the level of transcription.98 Deciphering how this process is perturbed in anemias due to ineffective erythropoiesis might allow the discovery of new effective compounds.
β-Thalassemia is the prototype of anemia that is due to ineffective erythropoiesis.31 After the encouraging results obtained thus far, long-term data on efficacy and safety are needed to better assess the value of luspatercept in the treatment of patients with β-thalassemia. Because parenchymal iron loading characterizes this condition, and iron restriction was shown to improve the efficacy of erythropoiesis in animal models, the findings of the ongoing trials evaluating TMPRSS6-LRx and VIT-2763 may be important to understand whether iron sequestration can lead to amelioration of anemia in patients with β-thalassemia. Combining iron sequestration with treatments specifically targeting ineffective erythropoiesis might be an interesting therapeutic strategy in the future.
MDSs range from indolent conditions lasting for years to forms approaching acute myeloid leukemia.74 The revised International Prognostic Scoring System (IPSS-R) allows us to distinguish between lower-risk MDS, with a median survival of approximately 6 years, and higher-risk MDS, with a median survival of 1.5 years and a high risk for leukemic evolution. Ameliorating anemia and quality of life is the main objective of treatment in lower-risk MDS, and a subset of patients with MDS-RS may benefit from treatment with luspatercept. However, the real-world effectiveness of this treatment is unclear, and real-life studies are needed to define it.
Acknowledgments
M.C.'s studies on ineffective erythropoiesis and myelodysplastic syndromes were supported by Associazione Italiana per la Ricerca sul Cancro and Fondazione IRCCS Policlinico San Matteo.
Authorship
Contribution: M.C. conceived this paper, reviewed the literature, and wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Mario Cazzola, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: mario.cazzola@unipv.it.
REFERENCES
Author notes
Edited by Associate Editor Mario Cazzola, this review series highlights 3 specific areas of scientific and clinical advances in understanding disorders of red cell production. Nobel laureate Gregg Semenza focuses on the hypoxia-inducible factor (HIF) pathway, from discovery through the understanding of its role in regulation of erythropoiesis to the potential of HIF inhibitors as therapy. In the second article, Caulier and Sankaran update the concepts of regulation of erythroid differentiation. Complementing these 2 articles, Cazzola provides a review on the clinical problem of ineffective erythropoiesis; this article highlights the interplay between inherited and acquired anemias due to ineffective erythropoiesis and iron-loading and the prospects for improved therapies.