Abstract
To enable effective oxygen transport, ∼200 billion red blood cells (RBCs) need to be produced every day in the bone marrow through the fine-tuned process of erythropoiesis. Erythropoiesis is regulated at multiple levels to ensure that defective RBC maturation or overproduction can be avoided. Here, we provide an overview of different layers of this control, ranging from cytokine signaling mechanisms that enable extrinsic regulation of RBC production to intrinsic transcriptional pathways necessary for effective erythropoiesis. Recent studies have also elucidated the importance of posttranscriptional regulation and highlighted additional gatekeeping mechanisms necessary for effective erythropoiesis. We additionally discuss the insights gained by studying human genetic variation affecting erythropoiesis and highlight the discovery of BCL11A as a regulator of hemoglobin switching through genetic studies. Finally, we provide an outlook of how our ability to measure multiple facets of this process at single-cell resolution, while accounting for the impact of human variation, will continue to refine our knowledge of erythropoiesis and how this process is perturbed in disease. As we learn more about this intricate and important process, additional opportunities to modulate erythropoiesis for therapeutic purposes will undoubtedly emerge.
Introduction to physiological erythropoiesis
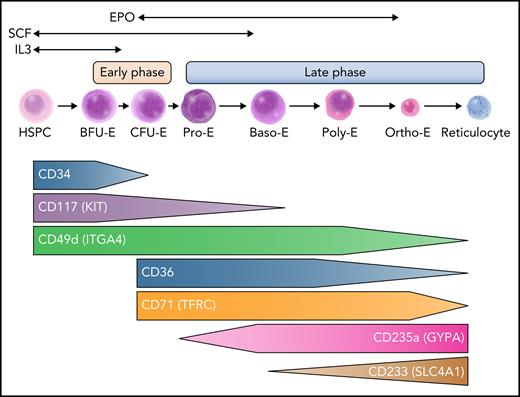
Each day, the process of red blood cell (RBC) production or erythropoiesis is crucial to maintain steady-state hemoglobin levels that allow for effective oxygen transport.1 This multistep process occurs in the bone marrow of adults in dedicated areas that cluster differentiating erythroid precursors around a central macrophage, a subcompartment in the bone marrow that has been termed the erythroblastic island.2 The erythroblastic islands enhance erythropoiesis by allowing cell-cell contacts to promote survival and proliferation, and the central macrophages engulf the extruded nuclei from erythroblasts. Erythropoiesis lasts ∼14 days in humans and includes 7 to 8 steps over 2 major phases (Figure 1). The earlier phase originates with multipotential hematopoietic stem and progenitor cells (HSPCs) that give rise to erythroid-committed progenitors, and the latter phase is characterized by maturation of erythroid precursors into enucleated reticulocytes that undergo terminal maturation into RBCs in the circulation. In the first phase, an initial group of erythroid-committed progenitors termed burst forming unit erythroid (BFU-E) cells are produced from multipotential HSPCs and subsequently differentiate into colony forming unit erythroid (CFU-E) cells. These progenitors are not morphologically identifiable but can be enumerated when they are cultured in semisolid media such as methylcellulose, allowing their identification a posteriori. Although BFU-Es and CFU-Es were once thought to represent distinct stages of progenitor cells, recent studies reveal heterogeneity in these populations and suggest a continuum of progenitor states.3 Subsequently, proerythroblasts are the first morphologically distinguishable cells and characterize the onset of the second phase of erythroid maturation. These cells then advance through multiple stages of differentiation distinguishable by morphology, including basophilic erythroblasts, polychromatophilic erythroblasts, and orthochromatic erythroblasts (Figure 1). Finally, the nucleus condenses and extrudes, giving rise to reticulocytes released into the peripheral blood where they fully mature into functional RBCs. A classical view of erythropoiesis involves differentiation and maturation through discrete and distinct stages as described; however, recent studies at single-cell resolution and using genetic approaches suggest a more continuous model of differentiation.4-7
An overview of human erythropoiesis. The early phase refers to the expansion of erythroid progenitors and is primarily sustained by interleukin-3 (IL-3) and stem cell factor (SCF). The late phase is dedicated to the maturation of erythroid precursors up to enucleation and is sustained by erythropoietin (EPO), although SCF continues to play a role in erythroid precursor expansion early in this phase. Throughout erythroid differentiation, the expression pattern of some surface markers changes and allows tracking by flow cytometry. Baso-E, basophilic erythroblast; BFU-E, burst-forming unit erythroid; CFU-E, colony-forming unit erythroid; HSPC, hematopoietic stem and progenitor cell; Ortho-E, orthochromatic erythroblast; Poly-E, polychromatophilic erythroblast; Pro-E, proerythroblast. Professional illustration by Somersault18:24.
An overview of human erythropoiesis. The early phase refers to the expansion of erythroid progenitors and is primarily sustained by interleukin-3 (IL-3) and stem cell factor (SCF). The late phase is dedicated to the maturation of erythroid precursors up to enucleation and is sustained by erythropoietin (EPO), although SCF continues to play a role in erythroid precursor expansion early in this phase. Throughout erythroid differentiation, the expression pattern of some surface markers changes and allows tracking by flow cytometry. Baso-E, basophilic erythroblast; BFU-E, burst-forming unit erythroid; CFU-E, colony-forming unit erythroid; HSPC, hematopoietic stem and progenitor cell; Ortho-E, orthochromatic erythroblast; Poly-E, polychromatophilic erythroblast; Pro-E, proerythroblast. Professional illustration by Somersault18:24.
As erythroid cells differentiate, the expression of many membrane surface antigens changes over time, allowing differentiation to be tracked and quantified by flow cytometry. It is possible to enrich for different subsets of erythroid progenitors based on CD34/CD36/CD71 and CD105 sequential expression in CD123 (IL-3 receptor) negative cells.8 Combining expression of GYPA (CD235A) and CD105, or alternatively band 3/SLC4A1 acquisition (CD233) and CD49d loss, can allow tracking of erythroid maturation (Figure 1).9 This cellular tracking can be helpful to elucidate the mechanisms of ineffective erythropoiesis.8,10 Importantly, although these surface markers enable enrichment of distinct stages of this differentiation process, sorted populations are still heterogeneous, and some subsets with intermediate antigen expression can be challenging to definitively classify.10 Conversely, emerging single-cell genomic studies suggest a more continuous view of this process, with a continuum of cell states that vary by global gene expression. Clustering of such data helps define specific erythroid subsets that seem to have distinct functional properties; it also highlights heterogeneity and substantial overlap within and between stages.3,5,7 It is likely that currently applied phenotyping approaches, although valuable to detect gross changes between each state of differentiation, may miss fine-grained alterations that can be revealed through these higher-resolution approaches. Nonetheless, many of the distinct stages identified have been functionally defined (eg, erythroid colony formation from BFU-Es), supporting a punctuated continuum model, where specific transitions occur across a continuum of overlapping states.4
Extrinsic regulation of erythropoiesis
A number of extrinsic factors control the differentiation, proliferation, and survival of erythroid cells by activating key downstream signaling pathways (Figure 1). Although not specific to erythroid differentiation, IL-3 has been shown to enhance the proliferation of early progenitors, including BFU-Es.11,12 Stem cell factor/KIT ligand binds the KIT receptor (KIT/CD117) and promotes the proliferation and survival of BFU-Es and CFU-Es, as well as proerythroblasts.13,14 The cytokine EPO is crucial for erythroid differentiation, allowing the survival and proliferation of maturing erythroid cells beginning with CFU-E progenitors through the later stages of maturation, although its receptor (EPOR) is weakly expressed on erythroid cells and quickly decreases with terminal maturation.15-18 In addition, EPO may also favor erythroid commitment from HSPCs, and EPOR may be more broadly expressed in progenitors than was once thought, supporting evolving models that reveal how erythroid lineage commitment may occur earlier in the hematopoietic hierarchy than initially envisioned.18-21 Identification and cloning of EPO have enabled the production of recombinant protein, which has significantly improved the treatment of anemic patients, particularly those with kidney or marrow failure.22-24 Other cytokines or soluble factors, such as insulin and insulin-like growth factor,25 transforming growth factor β superfamily members,26,27 and glucocorticoids, have been shown to modulate erythropoiesis28,29 as well as iron metabolism through the erythroferrone-hepcidin-ferroportin axis.30,31 We will not detail these pathways any further in this review, given prior extensive coverage of this topic.
All of the major cytokines and growth factors, including EPO, stem cell factor, and IL-3, stimulate a number of downstream effectors, including the JAK/STAT, MAPK, and phosphatidylinositol 3-kinase signaling pathways, which have been studied extensively, to promote survival and/or proliferation.32,33 For instance, EPO binding leads to EPOR dimerization that activates JAK2 through transphosphorylation. In turn, JAK2 phosphorylates STAT5, which promotes its nuclear translocation and helps shape the erythroid transcriptional program.34,35 Hyperactivation of these signaling pathways has been shown to promote erythrocytosis, most often through constitutive activation of JAK2 as a result of somatic mutations or less commonly through germline EPOR mutations.36-40 Older studies have portrayed these cytokines and growth factors as stimulating a fixed set of pathways. However, this canonical model has been expanded by newer studies using engineered molecules and naturally occurring genetic variation, revealing intricate and variable regulation of these downstream signaling pathways through modulation of receptor activity and downstream effectors.41-44 Better knowledge of these mechanisms is important to understand the control of erythropoiesis and illuminate how these pathways may be coopted in diseases states, as exemplified through the insights gained from new genes and pathways involved in erythrocytosis that can modulate these signaling mechanisms.45,46 These insights might lead to the development of targeted therapies to improve ineffective and defective erythropoiesis, as recently illustrated by the inhibitors of the transforming growth factor β receptor superfamily and modulators of the hypoxia-inducible factor pathway.26,47-49
Transcriptional control of erythropoiesis
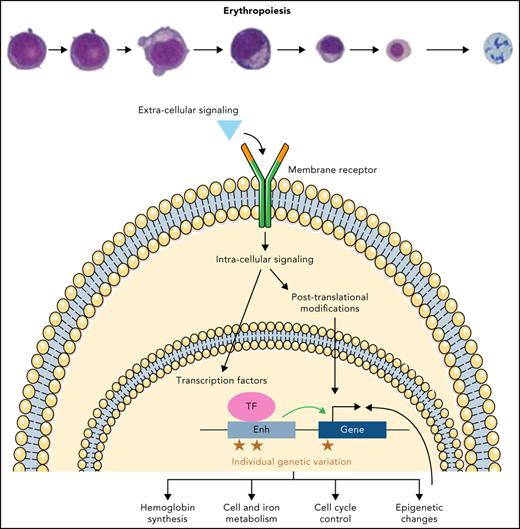
These extrinsic factors facilitate effective erythropoiesis by further activating downstream, intracellular pathways. These pathways converge in the nucleus, as exemplified by the activation of STAT5 downstream of JAK2 and EPOR, as discussed in the previous section. Moreover, the complex gene expression programs that characterize erythropoiesis are coordinated by a powerful set of intrinsically acting transcription factors (TFs),50 the potency of which is illuminated by their sufficiency to program nonerythroid cells into an erythroid fate.51 Key among these TFs is GATA1, which is critical to erythroid lineage commitment and differentiation and the expression of which is modulated throughout this process.52,53 Indeed, loss of GATA1 activity in mice and humans results in major impairments in erythropoiesis.54-59 GATA1 is highly expressed in erythroid cells from early progenitor stages, and its activity represses GATA2 during erythroblastic maturation. Although GATA1 expression slightly decreases after the proerythroblast stage proportionally to the global decrease in protein content throughout erythroblastic maturation, GATA2 expression is more dramatically reduced following this stage.60-62 This switch results in a strong increase in GATA1 activity, which in turn regulates numerous target genes that favor terminal erythroid maturation.63 The importance of direct competition between GATA2 and GATA1 is unclear; however, their binding sites widely overlap during erythroid differentiation, and cofactors including FOG-1/ZFPM1 and the nucleosome remodeling and deacetylase (NuRD) complex facilitate GATA1 binding to regulatory regions at the expense of GATA2.64-68 KLF1 is another key TF highly expressed in erythroid cells, the disruption of which leads to severe anemia when mutated in mice and causes a range of erythroid phenotypes in humans.69-71 KLF1 plays both an early role by favoring erythroid differentiation at the expense of megakaryocytic differentiation72,73 and a role in terminal maturation by triggering cell-cycle exit and chromatin condensation before enucleation.74-78 In addition, the roles of a number of other key TFs in erythropoiesis have been studied, although some of these factors, such as TAL1, LMO2, LDB1, and GFI1B, have a variety of roles in hematopoiesis.50,79
Although many TFs play vital roles in erythropoiesis, the work of single, isolated factors is insufficient to explain the complex changes that occur throughout this process. A more nuanced view of erythropoietic regulation recognizes these TFs function in complex networks, recruit and bind coactivators or corepressors to modulate their target gene expression, and also have altered interactions at different stages of this process (Figure 2).50,80 In the specific case of erythropoiesis, GATA1 binds coactivators, such as LMO2, LDB1, and TAL1/SCL, to activate the transcription of erythroid-associated genes.50 Conversely, GATA1 can recruit corepressors, including the polycomb repressor complex 2, which involves EZH2 and EED, the GFI1B/LSD1 complex, or the FOG-1/ZFPM1 and NuRD complex, to repress target genes that prevent erythroid differentiation.50,81-86 Moreover, corepressors seem to be more abundant and stable than coactivators, making the coactivators limiting factors in promoting erythroid differentiation.60 Each TF generally binds to distinct cis-regulatory elements, which are often enriched for TF-specific binding motifs and exhibit altered accessibility and other epigenetic changes during human erythropoiesis.63 The complexity of this regulation arises as a result of combinatorial activity at each cis-regulatory element for different TFs and for different cis-regulatory elements that synergistically (or at least additively) regulate gene expression, as exemplified by distinct regulation of key erythroid genes, such as the α- or β-globin gene clusters.4 Further complexity arises from the long-range chromatin interactions that underlie this process and that are essential for gene activation.87-89 Some TFs, including GATA1 and KLF1, primarily bind distal regulatory regions,68,90 but the precise regulatory logic involved remains poorly understood. For example, GATA1 is thought to bind proximal regions of induced genes, whereas it binds more distal regions of repressed genes, but these rules often vary at different genetic loci.91 Additionally, these TFs frequently recruit histone-modifying enzymes to specific regulatory elements that enable epigenetic alterations, which play critical roles in erythropoiesis.92-94
Models for control of erythroid gene expression by transcription factors and cis-regulatory elements. (A-B) The same cis-regulatory element can be bound by different TFs that exert antagonistic activities, such as GATA1and GATA2. (C) The same TF, such as GATA1, can either repress or activate gene expression, and this may depend, at least in part, on the distance of the cis-regulatory element to which it binds. (D) Different TFs, such as GATA2 and GATA1, can bind distinct cis-regulatory elements and control the same gene in an antagonistic manner. (E-F) The same TF, as exemplified by GATA1, can bind the same cis-regulatory element, but can display activating effects or inhibitory effects based on the recruitment of coactivators, such as the LDB1 complex, or corepressors, such as the GFI1B/LSD1 complex. These complexes help to activate or repress gene expression through epigenetic changes. (G-H) TFs can be modulated by long-range spatial interactions, bridging 2 distal enhancers through homo- or heterotypic interactions (with CTCF for instance) in a synergistic way. Professional illustration by Somersault18:24.
Models for control of erythroid gene expression by transcription factors and cis-regulatory elements. (A-B) The same cis-regulatory element can be bound by different TFs that exert antagonistic activities, such as GATA1and GATA2. (C) The same TF, such as GATA1, can either repress or activate gene expression, and this may depend, at least in part, on the distance of the cis-regulatory element to which it binds. (D) Different TFs, such as GATA2 and GATA1, can bind distinct cis-regulatory elements and control the same gene in an antagonistic manner. (E-F) The same TF, as exemplified by GATA1, can bind the same cis-regulatory element, but can display activating effects or inhibitory effects based on the recruitment of coactivators, such as the LDB1 complex, or corepressors, such as the GFI1B/LSD1 complex. These complexes help to activate or repress gene expression through epigenetic changes. (G-H) TFs can be modulated by long-range spatial interactions, bridging 2 distal enhancers through homo- or heterotypic interactions (with CTCF for instance) in a synergistic way. Professional illustration by Somersault18:24.
Genomic approaches have significantly advanced our knowledge of transcriptional regulation in a range of settings through chromatin occupancy analyses, assessment of accessible chromatin, and long-range interaction data, including in the context of erythropoiesis,95 but the development of improved technologies that enable such insights at single-cell resolution and with increasing precision (often at single-nucleotide resolution) is likely to further advance our knowledge of the precise mechanisms involved in TF regulation during erythropoiesis.96,97 Beyond the technologies that assess TF and chromatin alterations at increasingly higher resolution, the ability to more precisely manipulate this process, particularly using genome-editing tools, such as CRISPR/Cas9, is advancing our ability to define the functional roles of specific elements and even their subcomponents.98 This opportunity to manipulate specific cis-regulatory elements is likely to teach us a tremendous amount about the intricacies of gene regulation, as nicely exemplified by detailed mechanistic studies involving the regulation of the α-globin locus.99
Control of the erythroid cell cycle
Progression and variation in the cell cycle are crucial for erythropoiesis, because they balance proliferation and differentiation. Cell-cycle regulation is essential to determine the fate of early erythroid progenitors. Megakaryocytic-erythroid progenitors preferentially differentiate into erythroid progenitors as a result of a more rapid cell cycle arising from the activity of MYC, TP53, and the CDK4–cyclin D1 and CDK2–cyclin E complexes.100 Subsequently, there is a distinct transition involving a shortening of S-phase induced by the downregulation of p57KIP2 after the progenitor stages, allowing for the transition into proerythroblasts.101,102 Effective terminal erythropoiesis then requires cell-cycle exit, which requires many key factors, including RB1, E2F4, and CCND3 (cyclin D3).103-106 Interestingly, KLF1 deficiency in erythroid cells impairs enucleation after nuclear condensation, and orthochromatic erythroblasts continue to proliferate, which has been attributed to low levels of CDKN2C (p18) and CDKN1A (p21), which are required for cell-cycle exit.74
Posttranscriptional control of erythropoiesis
Although the major focus of studies on intrinsic regulatory mechanisms underlying erythropoiesis has been on transcriptional regulation, posttranscriptional mechanisms are also clearly important. It is striking that many of the key discoveries of these posttranscriptional processes arose from studies conducted in erythroid cells, including the discovery of ubiquitin-mediated protein degradation107 and early work examining the fundamentals of translational regulation by the ribosome.108 Despite limitations in routinely surveying these processes using genomic tools, which have resulted in a focus on transcriptional mechanisms over the past couple of decades, several important findings have emerged on the role of these posttranscriptional processes in erythropoiesis. It is clear that intricate regulation of ribosome levels is critical for altering protein production during hematopoiesis, including to enable erythroid lineage commitment through effective translation of GATA1 with higher ribosome levels in progenitors.109-111 These fundamental findings arose from studies of Diamond-Blackfan anemia, a rare bone marrow failure disorder that is characterized by a paucity of erythroid progenitors, with a majority of genetic mutations occurring in ribosomal proteins.112 Specific RNA binding proteins can also affect translation in erythroid cells, as exemplified by studies of the heme-regulated eIF2alpha HRI/EIF2AK1 kinase that plays critical roles in erythropoiesis by modulating messenger RNA translation.113,114
In addition to the intricate regulation of protein production, there are notable and dynamic changes that occur in the proteome during human erythropoiesis,61 including tremendous variation in protein levels at distinct stages of this process and dynamic alterations in posttranslational protein modifications, such as phosphorylation.115 Finally, the regulation of protein degradation has critical roles in enabling erythropoiesis. For instance, heat shock protein 70 (HSP70) promotes the survival of maturing human erythroblasts by protecting GATA1 from caspase-3–induced degradation, which is critical for erythroid maturation.116 Other heat shock proteins and chaperones help to maintain the conformation of nascent globin chains to facilitate their association and prevent aggregation in erythroblasts, which would lead to toxicity and ineffective erythropoiesis.117 Finally, there are a number of examples of how ubiquitin-dependent degradation through the proteasome can fine tune erythropoiesis. For instance, FBXO11-mediated degradation of the heterochromatin-associated protein BAHD1 is required to enhance erythroid transcription and enable effective erythropoiesis.118 Importantly, it is likely that current studies on translational and posttranslational protein regulation will significantly advance in the coming years, with improved methods for surveying translation, such as ribosome profiling,119 and through higher-resolution proteomic approaches.60,62,120 Importantly, integration of such studies with more holistic studies of erythroid cell biology is likely to reveal key changes during the process of erythropoiesis, such as the necessity of autophagy in enabling effective erythropoiesis to occur.121
How human genetic variation enables a deeper understanding of erythropoiesis
The regulatory mechanisms discussed in this article paint a stereotyped picture of human erythropoiesis; however, there is considerable interindividual variation in this process. Studies of this variation can provide key insights, both in the context of diseased states and in the context of healthy variation.122 Recent studies have revealed how common genetic variation found in the population can explain ∼15% to 25% of overall interindividual variation, and >8000 genetic variants have been associated with RBC traits.123 Importantly, this variation not only plays a role in healthy states, but also critically points to many genes that have crucial roles in erythropoiesis and diseases affecting this process, as exemplified by focused studies on specific loci harboring such variation.43,124 Large studies focusing on RBC indices have identified variation in many genes involved in monogenic RBC membrane defects including PIEZO1, ANK1, SLC4A1, monogenic enzymopathies including HK1 or PKLR, components involved in cytokine signaling, and regulators of iron metabolism.123,125 Some of these targets have been then functionally validated in vitro, as exemplified by SH2B3, the silencing of which resulted in increased RBC production, mimicking human erythrocytosis.43 Moreover, this common genetic variation underlies variable penetrance and expressivity among presumed monogenic blood disorders, and the combination of multiple common polymorphisms together might explain some RBC diseases that have been largely recalcitrant to monogenic mapping approaches, such as some forms of polycythemia, demonstrating how a spectrum of genetic variation likely underlies most, if not all, blood diseases.123
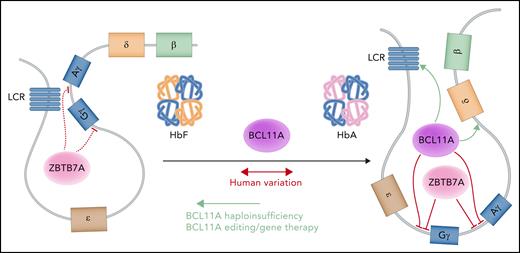
Perhaps the most important opportunity lies in the ability of genetic variation to shed light on critical biological mechanisms that can in turn enable more effective therapies in individuals with blood disorders. Much of the common genetic variation occurs in the noncoding genome and is likely to affect cis-regulatory elements that alter erythropoiesis. Although it is challenging to study such noncoding elements, there are important opportunities to gain functional insights into these putative regulators through dissection of the roles of these regulatory elements or their target genes.126 One of the earliest and most advanced examples of this directed approach characterized the TF BCL11A, which is now a key therapeutic target for fetal hemoglobin induction in β-hemoglobin disorders. Targeted suppression of BCL11A by gene therapy or genome editing approaches has shown clinical benefit (Figure 3).127,128 BCL11A was initially identified as a key transcriptional regulator of fetal hemoglobin production through functional follow-up of common genetic variant associations.129 Several subsequent observations then enabled effective therapeutic development and targeting of BCL11A. First, rare haploinsufficiency of BCL11A was shown to significantly elevate fetal hemoglobin levels, providing a key proof of principle for the effectiveness of targeting this factor in vivo in humans.130-133 Second, erythroid-specific enhancer elements of BCL11A were identified through follow-up of common variant associations and have helped to identify potent targets for genome-editing approaches.134,135 Third, clinical development of cutting-edge genome manipulation tools has enabled key opportunities to selectively perturb BCL11A expression.136 Although the early clinical studies are showing promise through targeting of BCL11A, it is important to further dissect the regulation mediated by BCL11A and other factors involved in fetal hemoglobin expression to define additional therapeutic approaches for the hemoglobin disorders (Figure 3).87,137-140 These findings, coupled with even further developments in our ability to manipulate the genome in a more precise manner or target molecules with greater effectiveness, should enable many future therapies. As more genetic studies are conducted, it will also be exciting to attempt to define additional therapeutic opportunities, particularly for red cell disorders that lack effective treatments at the current time.
A model of fetal hemoglobin (Hb) regulation. The upstream enhancer locus control region (LCR) preferentially interacts with the γ-globin genes to produce the γ-globin chains that assemble with α-globin chains to constitute the heterotetrametric fetal Hb (left). At baseline, ZBTB7A represses the γ-globin genes and helps maintain globin chain balance.87 To switch from fetal to adult Hb, BCL11A and ZBTB7A independently repress the transcription of the γ-globin genes to favor the interaction between the LCR and the β-globin gene (right), producing β-globin chains that associate with α-globin chains in the definitive molecule. BCL11A requires both proximal promoter and long-range interactions to carry out its activity. Professional illustration by Somersault18:24.
A model of fetal hemoglobin (Hb) regulation. The upstream enhancer locus control region (LCR) preferentially interacts with the γ-globin genes to produce the γ-globin chains that assemble with α-globin chains to constitute the heterotetrametric fetal Hb (left). At baseline, ZBTB7A represses the γ-globin genes and helps maintain globin chain balance.87 To switch from fetal to adult Hb, BCL11A and ZBTB7A independently repress the transcription of the γ-globin genes to favor the interaction between the LCR and the β-globin gene (right), producing β-globin chains that associate with α-globin chains in the definitive molecule. BCL11A requires both proximal promoter and long-range interactions to carry out its activity. Professional illustration by Somersault18:24.
Future outlook
Clearly, a significant amount has already been learned about the process of erythropoiesis and its regulation. Nonetheless, we continue to gain a refined view of this process with innovative approaches. For instance, single-cell genomic approaches have uncovered a continuum of states in human erythropoiesis, and it is likely that more studies will add further dimensions to this complexity. Another case is that of emerging technologies to survey key properties of the cell, such as variation in messenger RNA translation in single cells,141 which will undoubtedly extend key insights into how translational variation can affect human erythropoiesis.110 Important insights are likely to emerge as the field continues to study how human genetic variation affects this process. We have a fairly comprehensive parts list of regulators of erythropoiesis, cis-regulatory elements, and genetic variations affecting these elements; however, how all of these factors collectively act to regulate erythropoiesis remains mysterious; this is an important area that will likely yield significant fruitful insights in the coming years. Scalable genomic perturbation and functional approaches should enable improved insights, such as identifying the network of cis-regulatory elements that are regulated either by an individual TF or by a combination of TFs. Relating these networks to the ever-expanding set of human variants identified through genetic studies is likely to yield insights into how these variants can alter expression of specific genes and thereby contribute to the regulation of and interindividual variation in erythropoiesis.
At the same time, another area that is likely to see significant advances in the coming years is the study of clonal dynamics and its impact on erythropoiesis. Our understanding of erythropoiesis continues to evolve through single-cell genomic and functional assays.20,142 These newer approaches are beginning to provide fruitful insights into the pathogenesis of a variety of erythroid diseases. However, the clonal contributions to erythropoiesis are poorly understood. Bulk studies have shown that even oligoclonal hematopoiesis, as occurs in the premalignant state clonal hematopoiesis of indeterminate potential, can occur with ostensibly normal erythropoiesis.4,143 As improved tools to measure clonal dynamics and contributions in unmodified cells are developed, such as the assessment of nuclear or mitochondrial somatic mutations for phylogenetic inference, there will be many opportunities to dissect these clonal contributions at single-cell resolution.144,145 Our ability to discern whether pathological erythropoiesis can alter clonal contributions and increase the risk for clonal blood diseases, as illuminated by the myeloid malignancy predisposition that may occur in sickle cell disease, could be further enhanced with these advances.146 In many ways, although our understanding of human erythropoiesis can seem extremely advanced, as we learn more about this process, we are finding that there is just as much, if not more, that remains to be learned.
Acknowledgments
The authors apologize for the inability to comprehensively cover many key findings on human erythropoiesis and have focused on general overarching concepts. The authors are grateful to members of the Sankaran laboratory, particularly Rick Li and Richard Voit, for valuable comments and suggestions.
The laboratory of V.G.S. is supported by the New York Stem Cell Foundation, a gift from the Lodish family to Boston Children's Hospital, and National Institutes of Health grants R01 DK103794 (National Institute of Diabetes and Digestive and Kidney Diseases) and R01 HL146500 (National Heart, Lung, and Blood Institute). V.G.S. is a New York Stem Cell-Robertson Investigator.
Authorship
Contribution: A.L.C. and V.G.S. wrote the manuscript and designed the figures.
Conflict-of-interest disclosure: V.G.S. serves as an advisor to and/or has equity in Branch Biosciences, Ensoma, Novartis, Forma, and Cellarity, all unrelated to the present work. A.L.C. declares no competing financial interests.
Vijay G. Sankaran, Division of Hematology/Oncology, Boston Children's Hospital, 1 Blackfan Circle, Karp Family Research Building 7211, Boston, MA 02115 e-mail: sankaran@broadinstitute.org.
REFERENCES
Author notes
Edited by Associate Editor Mario Cazzola, this review series highlights 3 specific areas of scientific and clinical advances in understanding disorders of red cell production. Nobel laureate Gregg Semenza focuses on the hypoxia-inducible factor (HIF) pathway, from discovery through the understanding of its role in regulation of erythropoiesis to the potential of HIF inhibitors as therapy. In the second article, Caulier and Sankaran update the concepts of regulation of erythroid differentiation. Complementing these 2 articles, Cazzola provides a review on the clinical problem of ineffective erythropoiesis; this article highlights the interplay between inherited and acquired anemias due to ineffective erythropoiesis and iron-loading and the prospects for improved therapies.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal