Abstract
Hypoxia-inducible factors (HIFs) were discovered as activators of erythropoietin gene transcription in response to reduced oxygen (O2) availability. O2-dependent hydroxylation of HIFs on proline and asparagine residues regulates protein stability and transcriptional activity, respectively. Mutations in genes encoding components of the O2-sensing pathway cause familial erythrocytosis. Several small-molecule inhibitors of HIF prolyl hydroxylases are currently in clinical trials as erythropoiesis-stimulating agents. HIFs are overexpressed in bone marrow neoplasms, and the development of HIF inhibitors may improve outcomes in these disorders.
Molecular mechanisms of oxygen sensing
At any given moment, 20 to 30 trillion red blood cells (constituting ∼50% of all cells in the human body) are circulating through the vasculature of the average adult, whose bone marrow (BM) produces erythrocytes at the mind-blowing rate of 2 to 3 million cells per second.1 The major function of these erythrocytes is to ensure that every other cell in the body receives an adequate supply of oxygen (O2) to satisfy its metabolic demands, principally for mitochondrial respiration. The body does not count red blood cells; only hematologists do that. Instead, specialized cells, located primarily in the kidney, sense local O2 availability and respond to hypoxia by increasing their production of erythropoietin (EPO), which is secreted into the bloodstream and, in the BM, binds to its cognate receptor (EPOR) on the surface of erythroid precursor cells, triggering signal transduction pathways that increase erythroid cell survival, proliferation, and differentiation. The subsequent increase in red cell mass increases O2 delivery, thereby closing the loop on a beautiful homeostatic mechanism.
Thirty years ago, any mention of O2 sensing was accompanied by a wave of the hand and a quick transition by the lecturer to a slide summarizing the clinical indications for recombinant human EPO administration. This is not to say that investigators in the field lacked hypotheses2: one camp reasoned that because hemoglobin carried O2 in red cells, a heme protein might be involved in O2 sensing in the kidney; another camp theorized that because cytochrome c oxidase used O2 in complex IV of the mitochondrial electron transport chain, a cytochrome might be involved in O2 sensing. However attractive these hypotheses might have seemed, supporting data were neither abundant nor compelling.
The isolation of human EPO complementary DNA sequences3,4 was a breakthrough that enabled the synthesis of recombinant human EPO, thereby ushering in a new era in the treatment of anemia in patients with chronic kidney disease (CKD).5 It also enabled the isolation of human EPO genomic DNA sequences,3,4 which were required for subsequent studies of transcriptional regulation leading to the identification of sequences in the 3'-flanking region that were essential for hypoxia-induced EPO gene expression.6,7 Isolation of mouse Epo sequences8,9 led to the identification of a similarly situated hypoxia response element in the murine gene.10 Nucleotide-level resolution of the human EPO hypoxia response element led to the discovery of hypoxia-inducible factor 1 (HIF-1) as an activator of EPO transcription in cells cultured under low O2 conditions11 and the subsequent biochemical purification of HIF-1 from 120 L of HeLa cells grown in suspension culture,12 which enabled isolation of complementary DNA sequences encoding the HIF-1α and HIF-1β subunits.13 HIF-1α expression was O2-regulated: protein levels increased within 1 hour of hypoxic exposure and HIF-1α was completely degraded within 5 minutes of reoxygenation.14 Exposure of cells to O2 concentrations <6% O2 (corresponding to a partial pressure of O2 of ∼40 mm Hg at sea level) led to progressively increased HIF-1α protein levels.14 The demonstration that HIF-1 activity was induced in every mammalian cell line analyzed, whereas EPO was not, indicated a larger role for HIF-1 in responses to hypoxia.15
The trigger for degradation of HIF-1α under normoxic conditions was remarkably direct: the insertion of an O2 atom into a proline residue of the protein, which made HIF-1α irresistibly attractive to the von Hippel-Lindau (VHL) protein.16-18 VHL did not come alone to the party but brought a mob with colorful names like the elongins (B and C), CUL2, and RBX1.19 The outcome for HIF-1α was tragic; the protein was ubiquitinated and then liquidated by the proteasome.20,21 HIF-1α was marked for execution by a trio of HIF prolyl hydroxylase domain proteins (PHD1-3), which are dioxygenases that catalyze the insertion of one oxygen atom into Pro402 or Pro564 of human HIF-1α, while the second oxygen atom is used to decarboxylate α-ketoglutarate to succinate and carbon dioxide.22 The identification of α-ketoglutarate as a substrate of the HIF prolyl hydroxylases indicated that these enzymes could be targeted for inhibition by competitive antagonists.22 The PHDs have Fe(II) in their catalytic center, which provided a mechanistic basis for the induction of HIF-1 activity by Co(II) salts, such as CoCl2, and iron chelators, such as desferrioxamine, which also increase EPO levels.23
The ability of HIF-1α to activate transcription was also O2 regulated, independent of the effect on protein stability.24,25 FIH-1 (factor-inhibiting HIF-1) interacts with HIF-1α and inhibits its transactivation function26 by hydroxylating asparagine-803, thereby blocking interaction of HIF-1α with the coactivator protein and histone acetyltransferase p300.27 FIH-1 is another dioxygenase that uses O2 and α-ketoglutarate as substrates.28 Thus, both the stability and transcriptional activity of HIF-1α are negatively regulated by O2-dependent hydroxylation reactions that are inhibited under hypoxic conditions.
To sum up this section, starting with studies of EPO gene regulation, over the course of a dozen years, a small number of laboratories elucidated the fundamental molecular mechanisms controlling the expression of what are now recognized to be thousands of HIF target genes in every nucleated cell of just about every metazoan organism.29 The breakthrough discovery of HIF-1 opened the field of O2 biology to molecular analysis. In addition to many more HIF target genes, we also subsequently learned there were several more HIFs, namely, HIF-2α and HIF-3α, which are also O2 regulated, dimerize with HIF-1β, and activate transcription.30-32 As described in the next section, loss-of-function and gain-of-function mutations in mice and humans, respectively, have revealed that HIF-2α plays a critical role in regulating postnatal erythropoiesis.
Role of HIF pathway in familial erythrocytosis
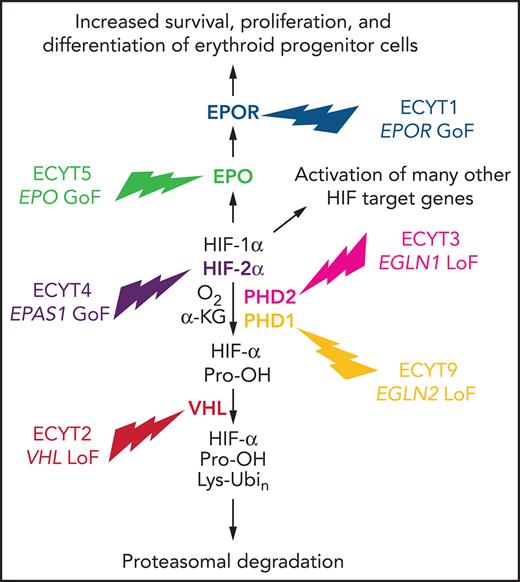
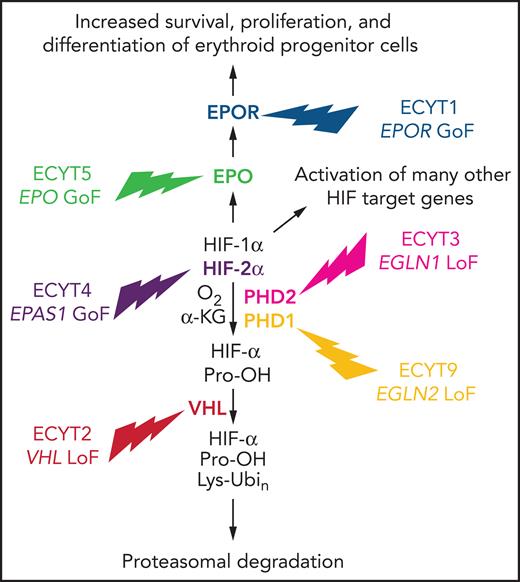
The elegant studies that delineated the O2-dependent regulation of HIF activity were performed using cultured cells in a dish. The question remained: how important was the HIF-PHD-VHL system for the regulation of EPO production and erythropoiesis in humans? The answer came from the genetic analysis of individuals affected by a rare Mendelian disorder known as familial erythrocytosis (ECYT). It is worth reminding the reader that in contrast to polycythemia, which is characterized by increased numbers of erythrocytes, platelets, and leukocytes, ECYT involves a selective increase in red cells only.
ECYT1 and ECYT5
The first affected individuals identified were found to be heterozygous for a mutation in the EPOR gene that truncated the carboxy terminus of the protein, leading to increased sensitivity of erythroid progenitor cells to stimulation by EPO.33-35 Whereas type 1 ECYT (ECYT1) is due to a gain-of-function mutation in EPOR, ECYT5 is caused by a gain-of-function mutation in the EPO gene that increases the rate of EPO protein synthesis.36 In each case, only a single mutant allele is required for the development of erythrocytosis, and disease occurs either as a result of de novo mutation or through inheritance of the mutant allele from an affected parent. The phenotypes of ECYT1 and ECYT5 are restricted to increased red cell production; in contrast, ECYT2-4 and ECT9 are characterized by pleiotropic manifestations resulting from systemic alterations in O2 sensing, as described in the remainder of this section. (ECYT6-8 result from mutations in the genes encoding β-globin, α-globin, and biphosphoglycerate mutase, respectively, that affect hemoglobin-O2 affinity and will not be discussed here.)
ECYT2
Erythrocytosis is endemic in the Chuvash region of the former Soviet Union and is inherited as a recessive trait; affected individuals are homozygous for an Arg200Trp missense mutation in the VHL gene that modestly impaired the interaction of VHL with hydroxylated HIF-1α or HIF-2α, leading to increased HIF transcriptional activity and target gene expression.37 Whereas EPOR and EPO gene mutations cause increased red cell production as an isolated finding, homozygosity for the VHL Arg200Trp allele causes both erythrocytosis with inappropriately elevated EPO levels38 and broad phenotypic manifestations, including augmented cardiac, ventilatory, and pulmonary vascular responses to hypoxia39 as well as exercise-induced changes in skeletal muscle energy metabolism.40 Analysis of gene expression in fibroblasts from affected individuals vs controls that were incubated at various O2 levels revealed that at every level tested, HIF target gene expression was increased in ECYT2 cells relative to control cells,39 as a result of reduced HIF ubiquitination and degradation.37 Affected individuals were found to have EPO levels that were either in the normal range or elevated, even though their hematocrits were greatly increased,38 suggesting that the former was responsible for the latter. The upward reset of normal red cell mass in these patients was illustrated by the response to phlebotomy: in response to a reduction of mean hematocrit from 66% to 48%, mean EPO levels rose from 51 to 121 U/mL (ie, despite the fact that postphlebotomy hematocrit was still at the upper limit of the normal range).38 Knock-in of the Arg200Trp mutation into the Vhl gene of mice proved that homozygosity for the mutation caused ECYT.41 Individuals with ECYT2 have an increased risk of arterial and venous thromboses, which are the leading causes of death38,42 and are independent of the degree of ECYT.43
Other recessive VHL missense mutations, including Asp126Asn, Pro138Leu, and His191Asp, were subsequently associated with increased red cell production and pulmonary hypertension in other populations.44-46 It is worth reminding the reader that in contrast to the systemic vasculature, which dilates in response to hypoxia, the pulmonary vasculature constricts in response to hypoxia in order to shunt blood flow away from areas of the lung that are not ventilated. However, global and persistent pulmonary arterial constriction leads to increased right ventricular pressure and hypertrophy, which ultimately results in heart failure and death.
The phenotype of ECYT2 should be distinguished from that of VHL syndrome, in which affected individuals are heterozygous for a germ line VHL mutation that severely impairs the function of the VHL protein; the other VHL allele is inactivated by somatic mutation, deletion, or methylation, leading to the development of the clear cell type of renal cell carcinoma (RCC) as well as retinal and central nervous system hemangioblastomas.47 These data suggest that the presence of two hypomorphic alleles is sufficient to prevent tumor formation in ECYT2, whereas the presence of a wild-type allele is sufficient to prevent ECYT in VHL syndrome. However, it is also possible that the Arg200Trp mutation is in linkage disequilibrium with another variant that is protective against tumor formation.42
ECYT3
The next gene to be identified as a site of mutation was the EGLN1 gene encoding the HIF prolyl hydroxylase PHD2. The PHD2 enzyme contains in its catalytic center an Fe(II) ion, which is coordinated by His313, Asp315 and His374, and the missense mutations Pro317Arg and Arg371His occur in close proximity to these critical residues, with Arg371 conserved in all metazoan HIF prolyl hydroxylases from human to worm.48,49 Since the original reports, a variety of frameshift, missense, and nonsense mutations have been identified that alter the catalytic domain in the carboxy-terminal half of the protein.50 A patient who was heterozygous for a germ line EGLN1 His374Arg missense mutation was found to have a paraganglioma (a tumor that arises from the sympathetic or parasympathetic chain ganglia) in which the wild-type EGLN1 gene was deleted, suggesting that in this tissue, EGLN1 functions as a tumor suppressor.51 Similar results were reported for a loss-of-function mutation of EGLN2,52 which encodes PHD1. EGLN2 mutations should be classified as ECT9 if other cases are reported.
ECYT4
ECYT4 is caused by heterozygous missense mutations in the EPAS1 gene encoding HIF-2α that replace Ala-530, Met-535, or Gly-537.53-55 These three residues are conserved in all vertebrate HIF-2α proteins and are adjacent to Pro-531, which is one of the two Pro residues at which HIF-2α is hydroxylated by PHDs. It is likely that each of these amino acid substitutions interferes with the hydroxylation of HIF-2α. Dominant inheritance was demonstrated in a family with 3 generations of affected individuals, who were all heterozygous for a Gly537Trp missense mutation.53 Patients with an EPAS1 Ala530Glu, Ala530Thr, or Ala530Val mutation have been reported with ECYT and paraganglioma.54,55 An EPAS1 Phe374Tyr mutation, which increased the half-life of HIF-2α protein, also caused both erythrocytosis and paraganglioma.56 Thus, either loss-of-function mutation of PHD1 or PHD2 or gain-of-function mutation of HIF-2α can result in increased red cell production and the development of paraganglioma.
In a family with 4 generations of affected individuals, who were heterozygous for an EPAS1 Gly537Arg mutation, several affected individuals were found to have pulmonary hypertension.57 In addition to an increased resting pulmonary artery pressure, breathing a hypoxic gas mixture resulted in a further augmentation of pulmonary artery pressure,57 as described previously for ECYT2. The finding that EPAS1 gain-of-function mutations increase red blood cell production was complemented by the finding that Epas1 conditional knockout in adult mice results in anemia.58 The finding that a VHL or EPAS1 mutation that increases HIF activity causes pulmonary hypertension was complemented by the finding that mice heterozygous for a knockout allele at the Hif1a59 or Epas160 locus are protected from the development of hypoxia-induced pulmonary hypertension.
The most well-known response to breathing a hypoxic gas mixture is an increase in respiratory rate. In contrast to individuals with ECYT2,39 individuals with ECYT4 did not manifest an augmented ventilatory response to hypoxia.57 A major difference between these two groups is that ECYT2 is characterized by increased levels of both HIF-1α and HIF-2α, whereas ECYT4 is associated with increased HIF-2α only, implicating HIF-1α in the respiratory response to hypoxia. However, it is also possible that the differing phenotypes may relate to altered VHL interactions with non-HIF proteins in ECYT2.
Taken together, genetic analysis of familial ECYT has identified mutations affecting components of the HIF-VHL-PHD pathway, demonstrating the essential role of these proteins in humans (and mice) for O2 sensing in general and erythropoiesis in particular (Figure 1). Given the overwhelming genetic evidence that increased HIF activity leads to increased erythropoiesis, does decreased HIF activity lead to decreased erythropoiesis in humans? When lowlanders sojourn to high altitude, they develop an erythrocytotic response. However, among Tibetan highlanders, the development of erythrocytosis is a hallmark (along with pulmonary hypertension and cognitive disorientation) of chronic mountain sickness, and many healthy Tibetans have the same hemoglobin level as lowlanders, despite living in an environment in which the air they breathe contains 14%, rather than 21% O2. Remarkably, the most highly selected genetic variants in the Tibetan genome map to the EPAS1 locus encoding HIF-2α and are associated with a blunted erythropoietic response to ambient hypoxia (reviewed by Semenza61).
Mutations in genes encoding HIF pathway components cause familial ECYT. A gain-of-function (GoF) mutation in the EPO or EPOR gene causes isolated ECYT, whereas a loss-of-function (LoF) mutation in the EGLN1, EGLN2, or VHL gene or a GoF mutation in the EPAS1 gene causes ECYT as part of a syndrome resulting from global dysregulation of the HIF pathway.
Mutations in genes encoding HIF pathway components cause familial ECYT. A gain-of-function (GoF) mutation in the EPO or EPOR gene causes isolated ECYT, whereas a loss-of-function (LoF) mutation in the EGLN1, EGLN2, or VHL gene or a GoF mutation in the EPAS1 gene causes ECYT as part of a syndrome resulting from global dysregulation of the HIF pathway.
Can breakthrough science yield a breakthrough therapy?
The demonstration that a single amino acid substitution in PHD2 resulted in sufficient loss of catalytic activity to cause increased EPO production and erythropoiesis and the discovery that the HIF prolyl hydroxylases use α-ketoglutarate as a substrate suggested that it should be possible to design low molecular weight competitive antagonists as a novel therapeutic approach to stimulate erythropoiesis in patients with anemia resulting from CKD. Two such HIF prolyl hydroxylase inhibitors (HIF-PHIs), roxadustat and vadadustat, have demonstrated efficacy as erythropoiesis-stimulating agents (and noninferiority to epoetin alfa or darbepoetin alfa, respectively) in phase 3 randomized clinical trials involving either dialysis-dependent or non–dialysis-dependent patients with CKD.62-65 The roxadustat trials involved patients with CKD in China,62,63 whereas the vadadustat trials involved patients in Europe and the United States,64,65 with a broad range of ethnic groups represented in the latter studies.
Unlike recombinant human EPO, which requires parenteral administration, these small-molecule HIF-PHIs are taken orally. Another benefit of HIF stabilizers, as they are also known, is improved serum iron availability through increased HIF-dependent expression of 5 proteins required for: (1) Fe(II) import from the intestinal lumen to epithelial cells, which is mediated by cytochrome b reductase and divalent metal transporter 1; (2) Fe(II) export from intestinal epithelial cells to the plasma by ferroportin; and (3) oxidation of Fe(II) to Fe(III) by ceruloplasmin for transport to the marrow by transferrin.66-70 HIF-PHIs also suppress production of hepcidin, thereby protecting ferroportin from degradation. The effect of HIF-PHIs on hepcidin production may be mediated by erythroferrone, which is a hormone produced by erythroid precursors in response to EPO stimulation,71 and/or by an erythroferrone-independent mechanism.72 The relationship between HIF activity and iron metabolism also involves a feedback mechanism, because iron chelation can induce HIF-α stabilization by inhibition of the HIF prolyl hydroxylases, which contain Fe(II) in their catalytic center, and HIF-2α messenger RNA (mRNA) contains an iron response element, which is bound by iron regulatory protein 1 to repress translation of HIF-2α mRNA into protein.73Irp1 knockout in mice causes increased red cell production and pulmonary hypertension,73,74 similar to the effect of EGLN1 or EPAS1 mutation in ECYT3 and ECYT4, respectively. Taken together, these beneficial effects of HIF-PHIs with respect to iron handling may obviate the need for IV iron administration in some patients with CKD.75
An added clinical bonus was the finding of decreased serum levels of total cholesterol, low density lipoprotein (LDL) cholesterol, and triglycerides in patients treated with roxadustat,62,63,76 which suggests, but does not prove, a cardiac benefit, especially because roxadustat also decreased high-density lipoprotein cholesterol levels, although the effect on LDL cholesterol was greater.76 It is not clear why vadadustat does not have a similar effect on LDL cholesterol levels.77 There are potential renal benefits of HIF-PHIs as well. A recent study showed that vadadustat treatment decreased serum urea nitrogen and creatinine levels and decreased expression of kidney fibrosis markers in a mouse model of CKD.72 Administration to healthy rats of roxadustat or molidustat, which is another HIF-PHI that is currently in phase 3 clinical trials, increased renal blood flow and glomerular filtration rate through a nitric oxide–dependent mechanism.78 These are exciting results that one can only hope will be replicated in clinical studies.
Now, for the bad news. As described in the previous section, individuals with ECYT2 have an increased risk of arterial and venous thromboses, which are the leading causes of death.38,42 The risk of thrombosis is independent of the degree of erythrocytosis, and was also observed in a family with ECYT4, implicating HIF-2 as playing a critical role in the pathogenesis of thrombosis.43 Although the roxadustat trials met their safety end points, there was a significantly increased incidence of thrombosis in patients treated with roxadustat as compared with patients treated with EPO, leading the US Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee to conclude in a live-streamed meeting on 15 July 2021 that the benefit-risk profile of roxadustat does not support approval for the treatment of anemia in patients with dialysis-dependent or nondependent CKD by votes of 12 to 2 and 13 to 1, respectively. Vadadustat is unlikely to fare better with the FDA, considering that it did not reach its primary safety end point in patients with non–dialysis-dependent CKD, which was time to first major adverse cardiovascular event.64 Additional studies are required to determine whether setting a lower target hemoglobin level will reduce the risk of adverse effects in patients with CKD treated with HIF-PHIs.
Role of HIFs in BM neoplasia
HIF activity is increased in many solid tumors, increased HIF-1α expression in primary diagnostic biopsy specimens is associated with increased patient mortality,79 and the HIFs activate expression of genes that play key roles in many aspects of cancer progression, including angiogenesis, cancer stem cell (CSC) specification and maintenance, metabolic reprogramming, immune evasion, invasion, and metastasis, as summarized in recent reviews.80-84 In addition to intratumoral hypoxia, HIF-1α expression is also induced by phosphatidylinositol 3-kinase (PI3K) → AKT → mammalian target of rapamycin (mTOR) signaling driven by epidermal growth factor receptor or HER2 protein kinase activity.85,86 Acriflavine was shown to block the heterodimerization of HIF-1α or HIF-2α with HIF-1β, thereby blocking HIF transcriptional activity, tumor angiogenesis, growth, and metastasis in mouse models.87,88 This section will focus on the role of HIFs in BM neoplasms.
ALL
HIF-1α and vascular endothelial growth factor (VEGF), which is an HIF target gene product,89 are overexpressed in childhood acute lymphocytic leukemia (ALL) cells compared with normal BM cells, and VEGF overexpression is associated with poor therapeutic response.90 Among 92 patients with pre–B-cell ALL stratified into quartiles by HIF-1α protein expression in BM biopsies (using reverse phase protein array), the survival of those with the highest HIF-1α levels was significantly decreased compared with those who had lower HIF-1α levels.91 Pre-B ALL cells, cultured under hypoxic conditions or transfected with an HIF-1α expression vector, are resistant to the growth-inhibitory effects of vincristine, methotrexate, or etoposide, whereas transfection with a locked antisense nucleic acid targeting HIF-1α mRNA restored the antiproliferative effects of these drugs.91 HIF-1α expression in pre-B ALL cells is induced in an mTOR-dependent manner by coculture with BM stromal cells.91 Deletion of HIF-1α severely reduces the frequency of leukemia CSCs in T-cell ALL.92
APL
Most patients with acute promyelocytic leukemia (APL) express an oncogenic fusion protein containing sequences from promyelocytic leukemia (PML) and the retinoic acid receptor α (RARA) genes and respond to combination therapy with all-trans retinoic acid (ATRA) and arsenic trioxide. The PML tumor suppressor protein was shown to inhibit HIF-1α and VEGF expression by inhibiting mTOR activity such that PML loss of function leads to HIF-1α overexpression,93 adding to a list of tumor suppressors that negatively regulate HIF-1α, which includes VHL, PTEN, and p53.79 Treatment of myeloid leukemia cells with ATRA increased HIF-1α expression and forced expression of HIF-1α enhanced leukemic cell differentiation induced by ATRA.94 APL with an alternative PLZF–RARA fusion protein is partially resistant to ATRA–arsenic trioxide, but coadministration of ATRA with PEG-SN38, a drug that inhibits HIF-1α expression, led to eradication of APL in preclinical models.95
CLL
Chronic lymphocytic leukemia (CLL) cells constitutively express HIF-1α and VEGF under normoxic conditions as a result of overexpression of microRNA 92-1, which binds to VHL mRNA, thereby blocking VHL protein synthesis.96 HIF-1 regulates the expression of genes encoding chemokine receptors and cell adhesion proteins that control interaction of CLL cells with the tumor microenvironment, and HIF-1α knockdown impairs CLL colonization of BM and spleen in mice.97 HIF-1α mRNA overexpression is an independent predictor of decreased overall survival in CLL.98 Treatment with the HIF inhibitor BAY87-2243 reduced BM infiltration by Eμ-TCL1 leukemia cells in transgenic mice.99
CML
Expression of HIF-1α and its target gene VEGF was induced in Ba/F3 cells by forced expression of BCR/ABL, the signature fusion protein tyrosine kinase of chronic myelogenous leukemia (CML), through PI3K-AKT-mTOR signaling.100 When these cells were selected for resistance to imatinib, an inhibitor of BCR/ABL kinase activity, resistance was dependent on BCR/ABL → HIF-1α signaling, which led to increased glycolysis and increased expression of transketolase, a key enzyme in the nonoxidative pentose phosphate pathway.101 HIF-1α is required for the maintenance of the CML CSC population,102,103 which were targeted by the HIF inhibitor acriflavine.104
AML
Hypoxia and HIF inducers trigger differentiation of acute myeloid leukemia (AML) cells.105 The AML1-ETO fusion protein (also known as runt-related protein 1) binds to HIF-1α and impairs its transcriptional activity in AML cells.106 HIF-1α conditional knockout was associated with accelerated tumorigenesis in 3 models of AML induced by retroviral expression of AML1-ETO9a, HoxA9-Meis1, or MLL-AF9.107 These observations all suggest that HIF-1 might inhibit the development of AML. However, it may have the opposite effect in established AML. In 84 cases of normal karyotype AML, increased HIF-1α protein expression demonstrated by immunohistochemistry of BM biopsy samples was associated decreased overall survival,108 as was also observed in AML/ETO1+ AML.109 Fms-related tyrosine kinase 3–internal tandem duplication is associated with resistance of AML cells to cytosine arabinoside, and forced expression of Fms-related tyrosine kinase 3–internal tandem duplication induced HIF-1α, leading to decreased expression of the equilibrative nucleoside transporter 1 that is required for cytosine arabinoside uptake.110 A marked expansion of hypoxic niches in BM has been observed after AML cell engraftment compared with engraftment of normal BM cells.111,112 AML CSCs localize to the endosteum, which is the most hypoxic region of BM.113-115 AML CSCs constitutively express HIF-1α, and treatment with the HIF inhibitor echinomycin eliminates AML CSCs,116 even in relapsed AML, with no adverse effect on normal hematopoietic stem cells.117 However, deletion of HIF-1α and HIF-2α was reported to have no effect on AML CSCs.118 Taken together, the data suggest that the role of HIFs in AML is highly context dependent.
MM
HIF-1α and HIF-2α are expressed in multiple myeloma (MM) plasma cells and associated with VEGF expression and vessel density, which in turn is associated with patient mortality.119 Forced expression of HIF-1α or HIF-2α in MM cells increases angiogenesis in tumor xenografts,120 whereas HIF-1α knockdown in MM cells decreases angiogenesis.121 The antiangiogenic effects of drugs used to treat MM, such as adaphostin, bortezomib, lenalidomide, and thalidomide, are due at least in part to HIF inhibition.122-125 HIF-1α knockdown by short hairpin RNA in MM cells inhibits the expression of multiple proangiogenic and proosteoclastogenic cytokines and dramatically decreases tumor burden and bone destruction.126
MDS
Myelodysplastic syndrome (MDS) is a clonal disorder characterized by ineffective hematopoiesis and lineage dysplasia of unclear pathogenesis. The median survival of patients with MDS is 4.5 years.127 Although new therapies have been approved, most patients relapse, with a subsequent median survival of <6 months.128 Increased HIF target gene expression was significantly enriched in BM from patients with MDS as compared with controls.129 HIF-1α protein expression was significantly increased in cKit+ BM cells from mice with knockout of Dnmt, Runx1, or Asxl, which model loss-of-function mutations observed in patients with MDS, although the mice do not develop frank MDS.129 Inducible overexpression of HIF-1α in blood cells of transgenic mice was sufficient to induce an MDS phenotype with the gradual development of leukocytopenia, macrocytic anemia, and splenomegaly and BM showing multilineage dysplasia.129 Treatment with the HIF inhibitor echinomycin reverted dysplastic BM cells and prolonged survival of MDS mice.129
MPNs
Myeloproliferative neoplasms (MPNs) include polycythemia vera, essential thrombocythemia, and primary myelofibrosis. A majority of patients with MPNs have a Val617Phe mutation in the JAK2 gene that causes constitutive JAK2 kinase activity, which drives increased myeloproliferation with increased PI3K-AKT-mTOR signaling and increased reactive O2 species, leading to increased HIF-1α protein synthesis and stabilization, and treatment with the HIF inhibitor echinomycin impaired colony formation by primary BM cells from patients with MPNs.130 Thrombosis is a major cause of morbidity and mortality in those with MPNs, and RNA sequencing of granulocytes and platelets identified HIF target genes with significantly altered expression in patients with MPNs vs controls or MPNs with thrombosis vs MPNs without thrombosis.131
Conclusion
The development of safe and effective HIF inhibitors is urgently needed for testing in the treatment of BM neoplasms as well as solid tumors. The use of HIF inhibitors as anticancer drugs will likely constitute the next breakthrough therapy generated by elucidation of the HIF pathway. Belzutifan, a HIF-2–selective inhibitor132 was recently approved by the FDA for the treatment of RCC and other tumors in patients with the VHL syndrome, in which the signature genetic lesion is VHL loss of function, and RCC progression is dependent on HIF-2α expression in association with HIF-1α loss of function. For hematopoietic neoplasms and solid cancers other than RCC, optimal therapy may require inhibition of both HIF-1 and HIF-2.
Acknowledgments
G.L.S. is the C. Michael Armstrong Professor at the Johns Hopkins University School of Medicine and an American Cancer Society Research Professor. Work in his laboratory is supported by grants from the American Cancer Society and the Armstrong Family Foundation.
Authorship
Contribution: G.L.S. is responsible for all aspects of this manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Gregg L. Semenza, Miller Research Building, Suite 671, 733 N. Broadway, Baltimore, MD 21205; e-mail: gsemenza@jhmi.edu.
REFERENCES
Author notes
Edited by Associate Editor Mario Cazzola, this review series highlights 3 specific areas of scientific and clinical advances in understanding disorders of red cell production. Nobel laureate Gregg Semenza focuses on the hypoxia-inducible factor (HIF) pathway, from discovery through the understanding of its role in regulation of erythropoiesis to the potential of HIF inhibitors as therapy. In the second article, Caulier and Sankaran update the concepts of regulation of erythroid differentiation. Complementing these 2 articles, Cazzola provides a review on the clinical problem of ineffective erythropoiesis; this article highlights the interplay between inherited and acquired anemias due to ineffective erythropoiesis and iron-loading and the prospects for improved therapies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal