Key Points
Eltrombopag and romiplostim are effective in older patients with ITP, with no fatal hemorrhages and 13.8% SROTs.
Thrombosis history and absence of secondary antithrombotic prophylaxis are associated with thromboses and recurrent events during therapy.
Abstract
The efficacy and safety of thrombopoietin receptor agonists (TRAs) in older patients with primary immune thrombocytopenia (ITP) are unknown. We investigated TRA response and switch, thrombotic/hemorrhagic risk, and sustained responses off-treatment (SROTs) in 384 patients with ITP aged ≥60 years. After 3 months, 82.5% and 74.3% of eltrombopag- and romiplostim-treated patients, respectively, achieved a response; 66.7% maintained the response (median follow-up, 2.7 years). Eighty-five (22.2%) patients switched to the alternative TRA; although no cross-toxicity was observed, 83.3% of resistant patients had a response after the switch. Thirty-four major thromboses (3 fatal) and 14 major hemorrhages (none fatal) occurred in 18 and 10 patients, respectively, while on TRAs and were associated with thrombosis history (subdistribution hazard ratio, 2.04, P = .05) and platelet count <20 × 109/L (subdistribution hazard ratio, 1.69; P = .04), respectively, at TRA start. A recurrent event occurred in 15.6% of patients surviving thrombosis, in all cases but 1 during persisting TRA treatment (incidence rate, 7.7 per 100 patient-years). All recurrences occurred in the absence of adequate antithrombotic secondary prophylaxis. Sixty-two (16.5%) responding patients discontinued TRAs; 53 (13.8%) patients maintained SROTs, which were associated with TRA discontinuation in complete response (P < .001). Very old age (≥75 years; 41.1%) was associated with the more frequent start of TRAs in the persistent/acute phase but not with response or thrombotic/hemorrhagic risk. TRAs are effective in older patients with ITP, with no fatal hemorrhages and with SROTs in a significant portion of patients. Caution is warranted in patients with a history of thrombosis, and a careful risk/benefit balance should be considered.
Introduction
Primary immune thrombocytopenia (ITP) is a rare acquired immune disorder that is characterized by a platelet count <100 × 109/L and increased bleeding risk.1-3 ITP has 2 incidence peaks: 1 in children and 1 in older persons. In the latter, the incidence of ITP increases from 1.94 to 4.62 per 100 000 in patients aged 60 to 75 years to 9 per 100 000 in patients older than 75 years of age.4-6 Consequently, the number of older patients with IT is substantial, and their management represents a major clinical challenge, because older age is associated with increased frailty, comorbidities, polypharmacy, and worse performance status.7 ITP has been reported to have a more aggressive course in older individuals, with an increased risk of bleeding at diagnosis, as well as thrombosis and infections during follow-up.8-12
Eltrombopag and romiplostim are thrombopoietin receptor agonists (TRAs) that increase platelet count by activating the c-mpl receptor, with subsequent enhancement of megakaryopoiesis,13,14 resulting in platelet responses in most patients.15-20 Despite their overall good safety profile, TRAs have been associated with an increased risk for thrombosis,21,22 particularly when additional cardiovascular risk factors are present.23,24
Little information is available on the selection criteria for eltrombopag and romiplostim, switch to the alternative TRA, and the possibility that discontinuing TRA therapy will maintain the response.25-28 Also, little is known about the timing, severity, and clinical management of vascular events that occur during TRA therapy. Addressing these areas of uncertainty is particularly relevant in older and much older individuals.23,29 Here, we present the results of a retrospective multicenter study including ITP patients treated with TRAs when aged ≥60 years that aimed to investigate the impact of older age on TRA selection, response, and switch; thrombotic and hemorrhagic risk associated with TRA therapy; and achievement of a sustained response off-treatment (SROT).
Materials and methods
Patients and study design
The ELDERLY-ITP-TRA observational retrospective study had the endorsement of the GIMEMA (Italian Group for Hematological Diseases of the Adult) ITP Working Party and included consecutive patients with primary ITP (simply referred to as ITP hereafter) who started TRA therapy according to standard clinical practice, in 21 Italian Hematology Centers (Appendix), at age ≥60 years. Patients who received concurrent corticosteroid therapy at the start of TRAs were included in this analysis. Clinical/laboratory data at diagnosis and during follow-up were reported in an electronic case report form that was developed to record all study data after deidentification of the patients with an alphanumeric code to protect personal privacy. After the first data entry, the follow-up information was validated with revision of clinical data, and specific queries were addressed to the centers in case of inconsistent data.
Any treatment decision was independent from participation in this study. Comorbidities were evaluated according to the Charlson Comorbidity Index (CCI),30 and cardiovascular risk factors (CVRFs) were recorded.31 Overweight was defined as body mass index ≥25 kg/m2. Thrombosis history, including acute myocardial infarction, transient ischemic attack/stroke, superficial vein thrombosis, major venous thromboembolism, and acute/chronic arterial obstructive disease. was also noted.
Definitions
ITP diagnosis was assessed according to standard criteria1 and defined as a platelet count <100 × 109/L in the absence of other causes. The term “chronic ITP” identified patients whose condition had lasted for ≥12 months.15 Responses to TRAs were graded according to current terminology,1 evaluated at 2 and 3 months from TRA start, and did not require any rescue medication during the preceding 4 weeks with concurrent resolution of bleeding signs.
Major thromboses included deep vein thromboses of the limbs, abdomen, and cerebral veins, pulmonary embolism, acute myocardial infarction, ischemic stroke, and peripheral arterial thrombosis, whereas minor thromboses included superficial vein thrombosis of the limbs, transient ischemic attack, and other venous thromboses. Progression of thrombosis was defined as an extension of thrombosis occurring within 3 months from the previous event; recurrences included thromboses of a site that was previously uninvolved or had interval documentation of partial/complete resolution of the first occlusion or had extension of thrombosis for >3 months from the previous event.
Adverse events (AEs) were graded according to Common Terminology Criteria for Adverse Events v4.0. Bleeding was defined as minor if grade 1-2 or major if grade 3-4.
TRA failure included a persistent thrombocytopenia (platelet count ≤30 × 109/L for 4 consecutive weeks) at the highest recommended dose, TRA discontinuation because of AEs, or death related to TRAs or ITP. Sustained response off therapy was defined as the time on response without any rescue medication from TRA discontinuation to response loss or to last contact.
Ethical aspects
The ELDERLY-ITP-TRA study (protocol code–ITP-2020-01) obtained approval from the Area Vasta Emilia Centro Ethics Committee and later was approved by the local Ethics Committee of all participating centers. Research was conducted in accordance with the Declaration of Helsinki. The study has no commercial support.
Statistical analysis
Statistical analysis was carried out at the Biostatistics Laboratory of the ITP Unit at the Institute of Hematology “L. and A. Seràgnoli” in Bologna. Continuous and categorical variables were summarized and compared as previously described. Risk factors for hemorrhage/thromboses during TRA therapy and the cumulative incidence of recurrent thrombosis were identified, considering death as a competing risk, from TRA start to the first event/last contact and from the index thrombosis to the recurrent thrombosis/last contact, respectively (Fine and Gray model). Incidence rates were compared using the exact mid-P estimation method. Overall survival and SROT were compared using the log-rank test (Kaplan-Meier function). Two-tailed P values <.05 were considered significant. Statistical analyses were performed using STATA Software, 15.1.
Results
Study population
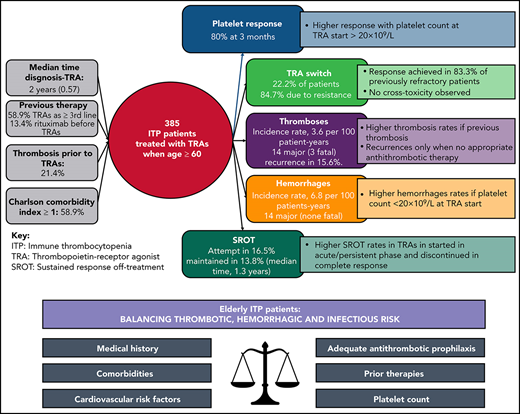
Overall, 390 older (age ≥60 years) patients, who were diagnosed with ITP in the 21 participating centers, started TRA treatment between February 2009 and April 2020. Six patients were excluded from the analysis: 4 for secondary ITP (3 associated with connective disease, 1 associated with low-grade lymphoma) and 2 for excess missing data. Therefore, the present analysis consists of 384 patients (Figure 1). All patients were followed until death or to data cutoff (1 November 2020).
Study flowchart. Numbers of individuals at each stage of the study, main descriptive results, and list of factors tested for association with outcomes.
Study flowchart. Numbers of individuals at each stage of the study, main descriptive results, and list of factors tested for association with outcomes.
The main demographic and hematological features of the study cohort are presented in Table 1. Median follow-up time from the TRA start was 2.7 years (range, 0.2-11.4), for a total observation of 3426 patient-years from ITP diagnosis and 1297 patient-years from TRA start. Seven (1.8%) patients received first-line TRAs because of uncontrolled diabetes (n = 3) or other comorbidities (n = 4) that discouraged high-dose corticosteroid and immunoglobulin therapy. Fifty-nine (13.4%) patients had received rituximab prior to TRAs. A total of 82 (21.4%) patients experienced ≥1 thrombosis prior to TRA. These thrombotic events occurred prior to ITP diagnosis (n = 60), between diagnosis and start of TRA therapy (n = 11), or prior to diagnosis and between diagnosis and TRA (n = 11). The most common comorbid conditions were diabetes (28.1%), solid tumors (16.2%), and acute myocardial infarction (12%). CCI was 0 in 41.2% of patients, 1 in 19% of patients, 2 in 9.6% of patients, and ≥3 in 30.2% of patients (supplemental Table 1, available on the Blood Web site).
Patients were stratified into 2 groups: without (CCI = 0) and with (CCI ≥1) comorbidities. Notably, CCI ≥1 was not associated with an increased incidence of hemorrhages at diagnosis or with a greater use of TRAs in the acute/persistent phase. Patients were also stratified according to age at start of the TRA (226 older patients [age 60-74 years], 58.9%; 158 much older patients [age ≥75 years], 41.1%). Notably, much older patients received TRAs more frequently as first- or second-line treatment (P = .002).
Choice of first TRA and platelet response
Eltrombopag was the first TRA used in 271 patients (70.6%), and romiplostim was the first used in 113 patients (29.4%). Although 3 hematological centers (18.8%) equivalently used eltrombopag or romiplostim, 11 centers (68.7%) used eltrombopag in >60% of the patients; only 2 centers (12.5%) preferentially began therapy with romiplostim (supplemental Figure 2). Compared with romiplostim, eltrombopag was started more frequently in older patients (P = .03).
The median platelet count at TRA start was 20 × 109/L (range, 1-50 × 109/L). At TRA start, 82 patients (21.4%) received concomitant ITP treatment (corticosteroids and/or immunoglobulins) for a median of 37 days (range, 5-120). Use of concomitant therapy was higher in much older individuals (27.2% vs 17.3% in patients <75 years; P = .02), but it was similar in eltrombopag- and romiplostim-treated patients (P = .97).
Most patients (85.6%) increased romiplostim from 1 μg/kg every week; in the remaining 15 patients, romiplostim was started at higher doses. Median duration of first TRA therapy was 1.2 years (range, 0.1-11.4) and was comparable across age groups (P = .97).
Overall, 255 (75.7%) of 337 and 264 (80%) of 330 evaluable patients achieved a platelet response at 2 and 3 months, respectively. Complete responses (CRs) were achieved in 42.7% and 46.7% of patients, respectively, at the 2 time points. The response rate at 2 months was higher in patients who received eltrombopag vs romiplostim (odds ratio, 1.78; 95% confidence interval [CI] 1.06-2.99; P = .03); however, at 3 months, responses to eltrombopag (82.5%) and romiplostim (74.3%) were comparable (P = .09). At 3 months, the response was higher in patients with a platelet count ≥20 × 109/L at TRA start (84.5% vs 75.3% in patients with lower platelet count; odds ratio, 1.79; 95% CI, 1.03-3.10; P = .04), but there was no association with age (P = .69), sex (P = .29), disease phase (chronic vs acute/persistent) (P = .07), CCI ≥1 (P = .47), or previous splenectomy (P = .76).
Overall, 66.7% of responding patients maintained the response until last contact, with a median response duration of 2.1 years, regardless of age (P = .12) and response at 2 months (CR vs response, P = .90).
Incidence and risk factors for TRA failure
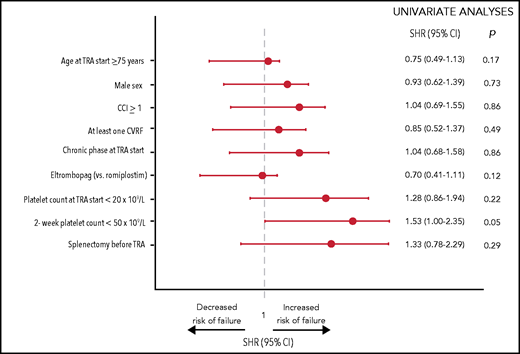
Overall, 96 patients (25%) failed TRA therapy. The cumulative incidence of failure was 18.4% at 1 year and was comparable in eltrombopag- and romiplostim-treated patients (supplemental Figure 3). Reasons for TRA failure were lack of response (n = 55; 57.3%) and TRA-related AEs (n = 41; 42.7%), specifically fluctuating platelet count (n = 11), transaminitis (n = 8), major hemorrhages (n = 2), major thromboses (n = 7; 3 were fatal), gastrointestinal disturbances (n = 5), skin rash (n = 5), and arthralgia (n = 3). In univariate analysis, only the absence of an early response tended to increase the risk of TRA failure (subdistribution hazard ratio [SHR], 1.53; P = .05; Figure 2).
Univariate analyses of risk factors associated with failure of TRAs. Risk factors for TRA failure were identified using the model of Fine and Gray, considering deaths and AEs unrelated to TRA treatment or ITP as competing risks. Single comorbidities and CVRFs were tested and were not associated with TRA failure: hypertension (P = .43), overweight (P = .50), dyslipidemia (P = .24), smoking (P = .60), diabetes (P = .63), solid neoplasia (P = .14), acute myocardial infarction (P = .23), peripheral vascular disease (P = .25), chronic kidney disease (P = .32), chronic obstructive pulmonary disease (P = .84), liver disease (P = .85), peptic ulcer (P = .42), congestive heart failure (P = .62), and transient ischemic attack/stroke (P = .68) (data not shown). Multivariable analysis was not carried out because only 1 covariate had a P value < .10 in univariate analyses.
Univariate analyses of risk factors associated with failure of TRAs. Risk factors for TRA failure were identified using the model of Fine and Gray, considering deaths and AEs unrelated to TRA treatment or ITP as competing risks. Single comorbidities and CVRFs were tested and were not associated with TRA failure: hypertension (P = .43), overweight (P = .50), dyslipidemia (P = .24), smoking (P = .60), diabetes (P = .63), solid neoplasia (P = .14), acute myocardial infarction (P = .23), peripheral vascular disease (P = .25), chronic kidney disease (P = .32), chronic obstructive pulmonary disease (P = .84), liver disease (P = .85), peptic ulcer (P = .42), congestive heart failure (P = .62), and transient ischemic attack/stroke (P = .68) (data not shown). Multivariable analysis was not carried out because only 1 covariate had a P value < .10 in univariate analyses.
Switch to the alternative TRA
Overall, 85 patients (22.2%) received both TRAs. The reasons for switching TRAs were lack/loss of response (n = 72, 84.7%; 19 of whom also experienced AEs) and AEs during response (n = 13, 15.3%). Median duration of first TRA therapy was 0.4 years, whereas the median time from stop of the first TRA to start of the second TRA was 1 day (range, 0-4.4 years), with most patients (65%) switching to the second TRA within 7 days. During the time interval between the 2 TRAs, 13 patients (15.3%) received rescue therapy with corticosteroids.
Among the 72 patients who were resistant to or relapsed after the first TRA, the second TRA achieved a response in 60 patients (83.3%; CR in 65.3%). Responses to the second TRA were not influenced by age (P = .37) or by type of TRA (P = .56). Overall, 37 of these 60 patients (61.7%) maintained the response until last contact. No cross-toxicity was observed in the 32 patients who experienced AEs during therapy with the first TRA.
Thromboses and hemorrhage during TRA therapy
A total of 43 thromboses in 35 patients was observed during or briefly after (≤14 days) TRA therapy, with 7 patients having ≥2 thromboses. The median time from TRA start to first thrombosis was 5.5 months (range, 0.3-67.2). Thromboses were arterial in 22 cases and major in 34 cases (79.1%), with 3 fatal events (acute myocardial infarction, ischemic stroke, pulmonary embolism). A total of 104 hemorrhages occurred in 65 patients (grade 2, 32.7%; grade ≥3, 13.5%), with 23 patients experiencing multiple events. No fatal hemorrhages were recorded.
Table 2 details the timing, frequencies, annualized incidence rates, cumulative incidences at 1 and 2 years, and types/sites/grades of thromboses and hemorrhage. The incidence rate of thromboses was higher during TRA treatment than before TRA start (3.6 vs 1.1 per 100 patient-years; P < .001), as well as when considering only major thromboses (2.9 vs 0.1 per 100 patient-years; P < .001).
The incidence rate of hemorrhage was higher during TRA treatment than before TRA start (6.8 vs 4.4 per 100 patient-years; P = .01), with no difference in the incidence rate of grade 3-4 events with or without TRA (1.1 vs 0.9 per 100 patient-years; P = .89). Antithrombotic treatment was associated with 14.4% (15/104) and 5% (6/120) of the bleedings that occurred during or before TRA treatment, respectively (P = .02). This association with antithrombotic therapy was no longer significant when considering only major hemorrhages (P = .26).
Risk factors for thrombosis and hemorrhage during TRA therapy
Among the 35 patients who had a thrombosis, 16 were males (median age, 73.7 years; range, 60-92.4). Twenty-eight patients (80%) had ≥1 CVRF, including hypertension (n = 22; 62.9%), overweight (n = 19; 57.1%), diabetes (n = 9; 25.7%), dyslipidemia (n = 7; 20%), and smoking (n = 3; 8.6%). CCI was ≥1 in 22 patients (62.9%). Eleven of 35 patients (31.4%) had a thrombosis prior to TRA start, and 12 (34.3%) were on antiplatelet/anticoagulant therapy at the time of thrombosis.
Among the 65 patients who had a hemorrhage during TRA therapy, 43.1% were male (median age, 72.4 years; range, 60-93); 12 patients (18.5%) were receiving antiplatelet or anticoagulant therapy.
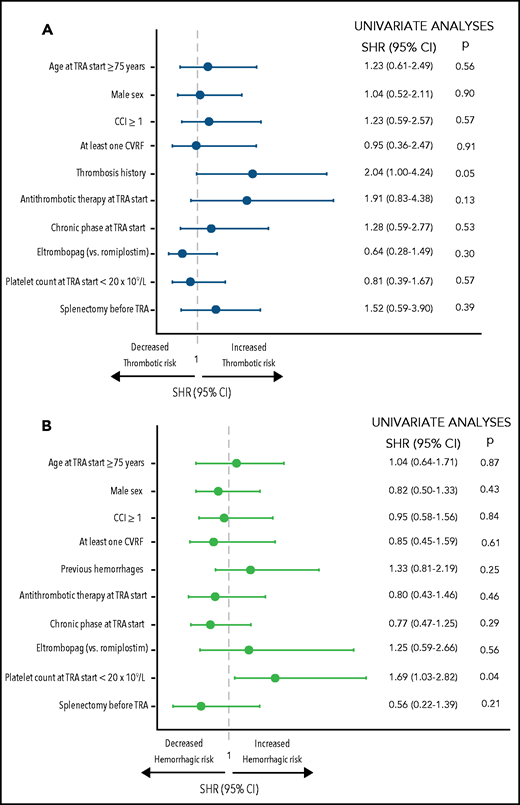
After adjustment for the risk of death, the cumulative incidence of thromboses and hemorrhage was not significantly associated with age (P = .56 and P = .87, respectively) or with type of TRA (P = .30 and P = .09, respectively). However, an association between thrombosis history and thrombosis of any grade during TRA was noted (SHR, 2.04; 95% CI, 1.00-4.24; P = .05; Figure 3A). This association remained statistically significant when considering major thromboses (SHR, 2.81; 95% CI, 1.14-6.92; P = .02).
Univariate analyses of risk factors associated with thromboses and hemorrhage of any grade. Risk factors associated with thromboses (A) and risk factors associated with hemorrhages (B) are reported. Single comorbidities and CVRFs were tested and were not associated with TRA failure: hypertension (P = .89), overweight (P = .61), dyslipidemia (P = .81), smoking (P = .67), diabetes (P = .97), solid neoplasia (P = .18), acute myocardial infarction (P = .22), peripheral vascular disease (P = .08), chronic kidney disease (P = .56), chronic obstructive pulmonary disease (P = .21), liver disease (P = .68), peptic ulcer (P = .46), congestive heart failure (P = .58), and transient ischemic attack/stroke (P = .39) (data not shown). Multivariable analyses were not carried out because only 1 covariate had a P value < .10 in univariate analyses.
Univariate analyses of risk factors associated with thromboses and hemorrhage of any grade. Risk factors associated with thromboses (A) and risk factors associated with hemorrhages (B) are reported. Single comorbidities and CVRFs were tested and were not associated with TRA failure: hypertension (P = .89), overweight (P = .61), dyslipidemia (P = .81), smoking (P = .67), diabetes (P = .97), solid neoplasia (P = .18), acute myocardial infarction (P = .22), peripheral vascular disease (P = .08), chronic kidney disease (P = .56), chronic obstructive pulmonary disease (P = .21), liver disease (P = .68), peptic ulcer (P = .46), congestive heart failure (P = .58), and transient ischemic attack/stroke (P = .39) (data not shown). Multivariable analyses were not carried out because only 1 covariate had a P value < .10 in univariate analyses.
On the other hand, hemorrhage of any grade was significantly higher in patients who started the TRA with a platelet count <20 × 109/L (SHR, 1.69; 95% CI, 1.03-2.82; P = .04) (Figure 3B). This association was confirmed when considering only major hemorrhages (SHR, 1.67; 95% CI, 1.02-2.71; P = .04).
No covariates predicted minor thrombotic or hemorrhagic events (data not shown).
Management of patients who experienced a thrombosis during TRA therapy and risk factors for recurrent thromboses
At the time of the first thrombotic event, 33 of 35 patients (94.3%) were in response (CR, 62.8%), whereas 2 patients had no response (5.7%). Thrombosis was fatal in 3 patients (8.6%). The median platelet count at the first thrombosis was 127 × 109/L (range 15-610 × 109/L). Overall, 5 of 32 patients (15.6%) discontinued the TRA immediately after the thrombosis because of a platelet count >300 × 109/L; 3 of these patients relapsed and received rescue therapy with corticosteroids, immunoglobulin, or mycophenolate mofetil. TRA therapy was continued with no dose adjustment or temporary interruption in the remaining 27 patients (84.4%).
Overall, 8 of 32 patients alive after thrombosis experienced progression or recurrence of thrombosis (7 of 27 patients who continued the TRA and 1 of 5 patients who discontinued therapy). More specifically, 3 patients (9.4%) experienced progression of thrombosis within 1 month from the clinical onset (after 2, 29, and 30 days), and 5 (15.6%) had a recurrence of thrombosis.
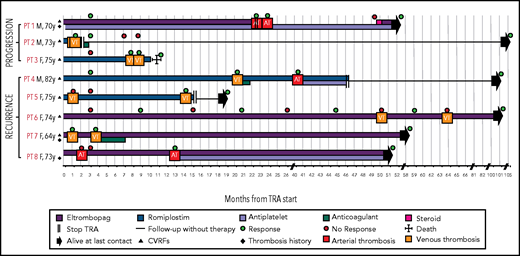
Figure 4 provides a graphical representation of the characteristics, therapy, and outcome of these patients. Patient #1 had progression of arterial thrombosis despite antiplatelet treatment. In the other patients, no antithrombotic treatment was given after the first thrombosis, with the exception of patient #4 who received a 1-month course of anticoagulation after VTE and had an arterial thrombosis 20 months later. Among the patients not receiving antithrombotic treatment, 2 had a progression of VTE (patients #2 and #3), 3 had a recurrent VTE (patients #5, #6, and #7), and 1 had a recurrent arterial thrombosis (patient #8). Patients #1 and #2 interrupted TRA therapy soon after the second thrombosis, with no further events. In the remaining patients, TRA therapy continued unchanged.
Graphical representation of the characteristics, therapy and outcome of the patients with progression of thrombosis or with recurrent thromboses. In patient #1, who discontinued the TRA at the time of first thrombosis, the second event occurred 30 days after TRA discontinuation. In patients #2 and #3, progression of thrombosis occurred after 2 and 29 days, respectively. Patient #3 died from renal cell carcinoma. Response: platelet count ≥30 × 109/L and at least a twofold increase over the baseline count. AT, arterial thrombosis; VT, venous thrombosis; F, female; M, male; PT, patient.
Graphical representation of the characteristics, therapy and outcome of the patients with progression of thrombosis or with recurrent thromboses. In patient #1, who discontinued the TRA at the time of first thrombosis, the second event occurred 30 days after TRA discontinuation. In patients #2 and #3, progression of thrombosis occurred after 2 and 29 days, respectively. Patient #3 died from renal cell carcinoma. Response: platelet count ≥30 × 109/L and at least a twofold increase over the baseline count. AT, arterial thrombosis; VT, venous thrombosis; F, female; M, male; PT, patient.
In the 5 patients with recurrent thromboses, the median time from first to second thrombosis was 13.8 months (range, 3.1-20). The incidence rate for recurrence in the 32 evaluable patients was 7.7 per 100 patient-years, and the cumulative incidence was 7.6% at 1 year. No significant association was detected between clinical/hematological features and recurrence of thromboses, including TRA discontinuation (data not shown).
Notably, in the 24 patients who had no recurrence or progression of thrombosis, 9 were on antithrombotic therapy at the time of the thrombosis and continued the treatment unchanged, whereas antithrombotic therapy was started after the thrombosis in 9 patients. Only 6 patients (25%) did not receive any antithrombotic therapy after the thrombosis. The rate of thrombosis progression or recurrence in the absence or presence of ongoing antithrombotic treatment was 46.7% (7/15) and 5.9% (1/17), respectively (P = .02).
Discontinuation and SROTs
Overall, 62 of 384 patients (16.5%) discontinued the TRA because of a stable response (55 patients during the first TRA and 7 after switching to the alternative TRA). The median time from TRA start to discontinuation was 0.9 years (range, 0.4-10.2). In 51 patients (82.3%), a tapering of TRA dose was performed before discontinuation. Incidence rates of TRA discontinuation because of a persistent response did not differ significantly between TRAs (0.5 and 0.8 per 100 patient-months in eltrombopag- and romiplostim-treated patients, respectively; P = .06). Notably, responding patients who discontinued TRA had started it during the chronic phase less frequently (43.5% vs 64.9%; P = .002).
At last contact, 53 (13.8%) patients maintained SROT, with a median duration of remission of 1.3 years (range, 0.03-9.6). In 9 patients, the response was lost after a median of 0.8 years (range, 0.06-7.8); all patients who restarted the previous TRA (n = 4) or switched to the alternative TRA (n = 4) achieved a stable response, with the exception of 1 patient who received steroid and azathioprine with no response at last contact. SROT was not affected by age (P = .89), sex (P = .80), type of TRA (P = .25), TRA start in chronic phase (P = .09), platelet count at TRA start (P = .91), or the presence of comorbidities (P = .35) or CVRFs (P = .36). TRA duration <6 months was not associated with SROT (P = .09). However, SROT was achieved more frequently in patients who discontinued TRA therapy with a CR (P < .001; Figure 5).
Treatment-free remission in patients who discontinued the TRA while in response, according to the response they had achieved (response [R] or CR). In patients who discontinued TRAs while in response, the duration of response also included the time spent in response without any rescue medication after TRA discontinuation. Responding patients who underwent splenectomy or discontinued TRAs because of AEs were censored at the time of TRA discontinuation. Median treatment-free remission was 28 months for patients whose response was not complete, whereas it was not reached in patients with a complete response. CR: platelet count ≥ 100 × 109/L; R: platelet count ≥30 × 109/L and at least a twofold increase over the baseline count.1
Treatment-free remission in patients who discontinued the TRA while in response, according to the response they had achieved (response [R] or CR). In patients who discontinued TRAs while in response, the duration of response also included the time spent in response without any rescue medication after TRA discontinuation. Responding patients who underwent splenectomy or discontinued TRAs because of AEs were censored at the time of TRA discontinuation. Median treatment-free remission was 28 months for patients whose response was not complete, whereas it was not reached in patients with a complete response. CR: platelet count ≥ 100 × 109/L; R: platelet count ≥30 × 109/L and at least a twofold increase over the baseline count.1
Overall survival
Overall, 43 patients (11.2%) died from nonischemic heart disease (13.9%), thromboses (11.6%: 3 patients during TRA therapy and 2 patients 12.3 and 27.5 months after TRA discontinuation), solid neoplasia (14%), age-related multiorgan failure (23.3%), and other non-ITP–related reasons, including liver cirrhosis, dementia, and renal failure (18.6%). Notably, 8 of 43 deceased patients (18.6%) died from infections, specifically bacterial lung infection (n = 3), pulmonary aspergillosis (n = 1), urinary tract infection (n = 1), peritonitis after peritoneal dialysis (n = 1), and sepsis of unknown origin (n = 2). The median age of these patients was 83 years (range, 68-93); only 1 patient had received rituximab.
There was no report of myelodysplastic syndrome, acute leukemia, or myelofibrosis.
Median survival time was not reached. At 3 years, overall survival was 89.8% and was significantly higher in patients aged <75 years (94.9% vs 82.1%; P = .002, log-rank test); it was not significantly influenced by sex (P = .09), type of TRA (P = .35), or platelet response at 2 months (P = .93) or 3 months (P = .14).
Discussion
The ITP cohort included in this multicenter study provides important information on the efficacy and safety of TRAs in older individuals, which is scarce to date. In much older patients, TRA administration was moved from the chronic phase to the persistent/acute phase and from third-line treatment to first/second-line treatment, possibly with the aim of reducing the toxicity of corticosteroids and accelerating platelet responses. This finding is consistent with 2 recent real-life studies that showed an earlier use of TRAs over the last decade, particularly in older individuals.9,29
In a recent retrospective Spanish study of 121 patients with ITP (median age, 63 years), romiplostim was used more often in patients with more severe hemorrhagic symptoms.25 We observed a preferential choice of eltrombopag in much older individuals, possibly because romiplostim self-injection or weekly hospital access is difficult for older patients.12 In addition to disease severity, patient characteristics, medical expertise, and hospital facilities (ie, dedicated staff/spaces for romiplostim administration) may influence the treatment strategy.
Overall, 75.7% and 82.5% of patients achieved a platelet response at 2 and 3 months, respectively. These rates are consistent with previous studies; however, they included a small number of older patients.9,18,22,23,29,32-36 The slower response to romiplostim may have been related to the time needed for uptitration; indeed, no significant difference in the 3-month response rate was noted between the 2 TRAs.37-39 Age also did not have a significant impact on the toxicity-related discontinuations that occurred in 10.7% of patients after a median treatment duration of 1.2 years. Although not negligible, this figure may be acceptable in a frail cohort. In the RAISE and EXTEND trials with eltrombopag, in which the median age of patients was 47 and 50 years, respectively, toxicity-related discontinuation occurred in 9.6% and 14%, respectively, after a median follow-up of 0.5 and 2.37 years.17,18,32 In the phase 2 trial and in the open-label extension study (median age, 52 years; median follow-up, 2.1 years) with romiplostim in ITP, 3.6% and 3.8% of patients discontinued treatment because of AEs, respectively.19,32 Notably, the positive impact of early response on a decreased risk for treatment failure may deserve validation in larger cohorts.
Annualized thrombosis rate in adults treated with TRAs is reported to be 2 to 3 times higher than in untreated ITP populations and in healthy matched individuals, representing 1 of the main concerns with TRA use.18,21,24,32,40 Our study, lacking a control arm, cannot determine whether and to what extent TRAs may increase the risk of thrombosis; however, the incidence rate of thromboses during TRA treatment (3.6 per 100 patient-years) was higher than before the start of TRA treatment, which aligns with current knowledge.24 Indeed, in the long-term studies, the incidence rate of thrombosis was estimated to be 4.16 per 100 patient-years in patients receiving romiplostim and 2.53 per 100 patient-years in patients receiving eltrombopag.24
Older age is a known risk factor for thrombosis and is associated with increased thrombotic risk in ITP.9,41,42 In a recent cooperative retrospective study of 451 older patients, including 134 individuals treated with TRAs, the rate of thrombosis was 1.7 per 100 patient-years; it was increased significantly by the presence of CVRFs and previous thromboses but not by TRA use.23 Here, a thrombosis occurred in 9.1% of patients, with 3 fatal cases, and 25% of living patients experienced progression of thrombosis or a recurrent event. The high incidence of progression or recurrence in patients with severe thrombocytopenia or under antiplatelet/anticoagulant therapy may be due to the ITP intrinsic thrombophilic state,24 as well as to the advanced age of this cohort and the presence of comorbidities/CVRFs in many patients. In the present cohort, a thrombosis occurred prior to diagnosis of ITP in 71 of the recruited subjects (18.2%). This prevalence of thrombosis before diagnosis of ITP in this older population is definitely lower than the lifetime thrombotic risk reported in the general population,43,44 so we can exclude a selection bias. However, a history of thrombosis is a significant risk factor for developing thromboses during TRA treatment. No recurrences occurred in the only patient who discontinued the TRA at the time of the first thrombosis. Conversely, patients who continued TRA therapy experienced progression and recurrences. Notably, almost all of those patients (7/8) did not receive long-term antithrombotic treatment after the first event; on the other hand, in patients continuing TRAs without progression or recurrence of thrombosis, an antithrombotic treatment was prescribed in 75% (18/24). Therefore, in the case of TRA-associated thrombosis there is a relative contraindication to continuing TRA, but antithrombotic treatment could modify the risk/benefit balance, allowing a proper secondary prophylaxis and the safe continuation of TRA treatment. These considerations are particularly relevant in patients with a supranormal platelet count at the time of thrombosis. In our cohort, all patients with a platelet count >300 × 109/L discontinued the TRA at the time of the first thrombosis; however, 1 patient experienced recurrent thrombosis.
Overall, bleeding events during TRA treatment were more frequent than thromboses. A nonnegligible portion of bleeding events that occurred during TRA treatment (14.4%) was associated with antithrombotic treatment; therefore, implementation and standardization of thrombotic and bleeding risk assessment should be pursued for a better management of these patients. However, the reduction in the mortality rate from bleeding to 0 is a very relevant figure, especially compared with previous studies on older patients not treated with TRA, in which 1.3% to 2% died from bleeding.10,11,45
Finally, we observed that only a small portion of patients received rituximab therapy before TRAs, possibly because the anti-CD20 antibody is associated with lower response rates and higher rates of infection in older individuals.46 The balance between the risk of thrombosis from TRA vs the risk of infection with rituximab requires careful evaluation of medical history and comorbid conditions, with case-by-case clinical decisions. From a practical point of view, in much older individuals with no history of CVRF or thrombosis, TRAs may be more attractive. On the other hand, patients at high thrombotic risk may be more suited for rituximab therapy, particularly if prolonged corticosteroid therapy is avoided, thus decreasing the overall infectious risk.
The efficacy of the switch between the 2 TRAs in case of resistance or intolerance has been described in adult ITP,27,47 but no information is available in older individuals. Overall, 22% of patients switched to the alternative TRA. A platelet response was achieved in 83% of previously refractory patients and was maintained in >60% of the cases. Because responses occurred regardless of age, switching to the alternate TRA should also be considered as the first option in much older individuals.
In younger populations, TRA discontinuation was attempted in 15% to 43% of patients.48-54 Possibly as the result of a reluctance to discontinue TRAs in older individuals, TRA discontinuation was attempted in a relatively small subset (16.5%) of our cohort. However, of these 62 patients, 85.5% (representing 13.8% of the total cohort) maintained an SROT, with the highest probability of SROT when the TRA was started in the acute/persistent phase and discontinued during CR. Recently, an Italian phase 2 study investigated the proportion of acute/persistent ITP patients that could achieve an SROT after treatment with eltrombopag for 24 weeks.26 SROT was observed in 38% responders and 25% evaluable patients and tended to be more frequent in complete responders, as observed here and in other settings.55 Overall, the degree of response is an important factor to be considered when evaluating treatment discontinuation.
The main limitation of this study is its retrospective nature, which may include underreporting or overreporting of events. Nonetheless, the substantial number of patients, the cooperation of centers with a particular focus on ITP, and the accurate revision of each case history may compensate in part for these intrinsic shortcomings. Of course, this limitation cannot be avoided when dealing with a rare condition, such as ITP, and a specific subpopulation, such as older patients. On the other hand, after the approval of TRAs for ITP therapy, retrospective studies may represent the only valuable source of comprehensive data.
Overall, these findings provide important real-life evidence that TRAs may be effective and safe in older patients, with no fatal hemorrhages and with successful SROTs in a nonnegligible portion of patients. The risk of thrombosis remains considerable. The risk-benefit balance between thrombotic and bleeding events deserves a careful evaluation of the risk factors for both events before prescribing TRAs. Further studies are needed to explore whether antithrombotic prophylaxis could be considered in ITP patients with a clear indication for TRAs, in the presence of a high thrombotic risk and no risk factors for bleeding other than ITP. Finally, the continuation of the TRA should not be discouraged after a thrombotic event if it may allow the safe start of secondary antithrombotic prophylaxis.
Acknowledgments
This work was supported by Associazione Italiana contro le Leucemie-Linfomi e Mieloma Bologna and conducted within the framework of GIMEMA (Gruppo Italiano per le Malattie Ematologiche dell’Adulto:Italian Group for Haematological Diseases of the Adult).
A complete list of the participating centers appears in “Appendix.”
Authorship
Contribution: F.P. N.V., V.D.S., and F.R. designed research; all authors performed research and collected data; D.B. performed statistical analyses; and F.P. N.V., V.D.S., and D.B. analyzed and interpreted data and wrote the manuscript.
Conflict-of-interest disclosure: F.P., W.B., and E. Rivolti have acted as consultants and received honoraria from Novartis. M.G.M. has received honoraria from Amgen, Bayer, CSL, Pfizer, Roche, Shire, and Sobi. F.R. has received honoraria from Amgen, Argenx, and Novartis. M Cavo has acted as a consultant and received honoraria from Janssen, Bristol Myers Squibb, Celgene, Sanofi, GlaxoSmithKline, Takeda, Amgen, Oncopeptides, AbbVie, Karyopharm, and Adaptive Biotechnologies. F.Z. acted as consultant and received honoraria from Novartis, Amgen, Grifols, Roche, Janssen Cilag, Takeda, Gilead, BMS, Sandoz. V.D.S. has received consulting and speaker fees from Alexion, AOP Orphan Pharmaceuticals, Amgen, Celgene, Grifols, Novartis, Sanofi, Sobi, and Takeda and has received research grants from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Valerio De Stefano, Section of Hematology, Catholic University School of Medicine, Fondazione Policlinico Gemelli IRCCS, Largo Gemelli 8, 00168 Rome, Italy; e-mail: valerio.destefano@unicatt.it.
Data sharing requests should be sent to Valerio De Stefano (e-mail: valerio.destefano@unicatt.it).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Appendix: participating centers
The following centers participated in this study: Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli”/Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale, Università di Bologna, Bologna (Francesca Palandri, Nicola Vianelli, Michele Cavo, Daniela Bartoletti, Giuseppe Auteri, Emanuele Sutto, Christian Di Pietro, Federica D’Ambrosio, Stefania Giaquinta); Section of Hematology, Department of Radiological and Hematological Sciences, Catholic University School of Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome (Valerio De Stefano, Elena Rossi, Angela Maria Ciminello, Sara Ceglie, Francesca Di Landro); Hematology, Department of Translational and Precision Medicine, Sapienza University, Rome (Antonietta Ferretti, Maria Gabriella Mazzucconi); Hematology Project Foundation, affiliated with the Hematology Department of the San Bortolo Hospital, Vicenza/Hematology Division, San Bortolo Hospital (Francesco Rodeghiero, Marco Ruggeri, Giuseppe Carli); Struttura Complessa (SC) Ematologia, Azienda Sanitaria Universitaria Giuliano Isontina, Trieste (Elisa Lucchini); Hematology Department, Careggi University Hospital, Florence (Valentina Carrai); Hematology Unit, Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan (Wilma Barcellini, Juri Alessandro Giannotta); Divisione di Ematologia, Azienda Ospedaliero-Universitaria (AOU) Maggiore della Carità di Novara e Dipartimento di Medicina Translazionale, Università del Piemonte Orientale, Novara (Andrea Patriarca, Maura Nicolosi, Paola Boggione, Riccardo Moia); Department of Hematology, Azienda Unità Sanitaria Locale (AUSL)–IRCCS–Reggio Emilia (Elena Rivolti, Katia Codeluppi); Unità Operativa Complessa (UOC) di Ematologia, Azienda Ospedaliera di Rilievo Nazionale e di Alta Specializzazione (ARNAS)–Garibaldi di Catania, Catania (Ugo Consoli, Donatella Calogero); Dipartimento di Ematologia e Oncologia, Niguarda Cancer Center, Grande Ospedale Metropolitano Niguarda, Milan (Silvia Cantoni); Hematology Unit, Grande Ospedale Metropolitano Bianchi Melacrino Morelli, Reggio Calabria (Esther Natalie Oliva); Department of Hematology and Bone Marrow Transplantation, University Hospital Federico II, Naples (Federico Chiurazzi, Martina Calabrò, Francesco Muriano); Ematologia, Ospedale Businco, Università degli Studi di Cagliari, Cagliari (Giovanni Caocci, Maria Pina Simula, Olga Mulas); Division of Hematology, Centro di Riferimento Regionale per la Prevenzione, Diagnosi e Cura delle Malattie Rare, AOU Policlinico Vittorio Emanuele, Catania (Gaetano Giuffrida, Daniela Nicolosi, Uros Markovic, Annalisa Condorelli, Marina Parisi); Hematology Division, Centro di Riferimento Regionale Malattie Emorragiche e Trombotiche dell’adulto, Città della Salute e della Scienza Hospital, Turin (Alessandra Borchiellini, Jacopo Agnelli); Hematology, University Hospital Policlinico Umberto I, Rome (Erminia Baldacci, Cristina Santoro); Hematology Division, Ospedale San Gerardo, ASST Monza, Monza (Monica Carpenedo); and Department of Medical, Surgical and Health Sciences, University of Trieste, Trieste (Francesco Zaja).


![Treatment-free remission in patients who discontinued the TRA while in response, according to the response they had achieved (response [R] or CR). In patients who discontinued TRAs while in response, the duration of response also included the time spent in response without any rescue medication after TRA discontinuation. Responding patients who underwent splenectomy or discontinued TRAs because of AEs were censored at the time of TRA discontinuation. Median treatment-free remission was 28 months for patients whose response was not complete, whereas it was not reached in patients with a complete response. CR: platelet count ≥ 100 × 109/L; R: platelet count ≥30 × 109/L and at least a twofold increase over the baseline count.1](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/138/7/10.1182_blood.2021010735/6/m_bloodbld2021010735f5.png?Expires=1769405918&Signature=kvYQh8lDC1tWxlxBUIn28-KfF4ocA76vLGTpZiGaPj9WymrF2hDcc01YSGh4r3cRXr76prCtxqQ7rtfBh3u7BHxoosbWaQ-zjNdi8ryrxXNKwVdP3Dn9o5sdTKhnHcif94~oqqsvGPyaXnElR29YZ0xWieI-YiF0GD9~gNBOfs-ZLmQZOymg7S2fmQrqbwsQWr2LxPvvswwkLG1b1JPKGCy~ZTiOG5eJAk9DauN~4PFOwjrNlIfcjld0q3Ky8CIVSMTMj7rE8t1r59TzVIEmgCQhFVkPRrTnldLjNBD5vl5We7FCJ-lo7d7lf6eAtKgNHCrevFiAouDZaCaLe-EgBg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
