Abstract
Acute graft-versus-host disease (GVHD) is a major life-threatening complication after allogeneic hematopoietic cell transplantation (allo-HCT). The classical target organs of acute GVHD include the intestines, liver, and skin. The damage of these organs is relatively easy to detect for the clinician as diarrhea, increased bilirubin, and rash. However, there is increasing evidence that other organs, where the acute damage is less apparent or more difficult to distinguish from drug toxicity, such as the central nervous system, lungs, ovaries and testis, thymus, bone marrow, and kidney, can be target organs of acute GVHD. Here, we review current evidence for nonclassical manifestations of acute GVHD in rodent models and in patients and discuss them in the context of novel emerging therapies for GVHD. A better understanding of the involvement of nonclassical GVHD target organs may help to improve patient outcomes after allo-HCT.
Introduction
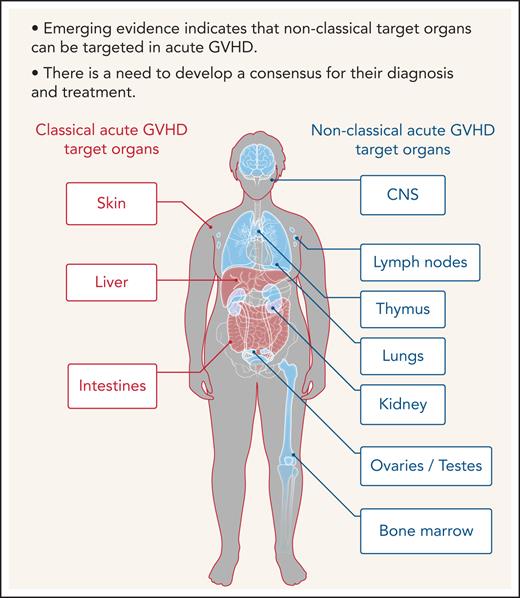
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative therapy for a variety of conditions, including hematologic disorders, metabolic storage diseases, immune deficiencies, and hematologic malignancies.1 Acute and chronic graft-versus-host diseases (GVHDs) are major hurdles that need to be overcome to make allo-HCT more safe.2,3 Skin, intestines, and liver are the predominant sites of acute GVHD, but many organs may be affected. The predominant involvement of the skin, intestinal tract, and liver was defined as the “tissue tropism of acute GVHD.” Chronic GVHD affects a variety of tissues and organs more frequently compared with acute GVHD. Tissue tropism in diseases can be determined by multiple factors that are released in an inflammatory microenvironment. Regulation of effector cell migration into target tissues occurs in a complex milieu of chemotactic signals where several receptors may be triggered simultaneously or successively. Chemokines expressed in inflamed tissues cause the recruitment of effector cells, such as T cells, neutrophils, and monocytes.4 On stimulation, alloreactive, donor-derived T cells switch chemokine receptor expression and acquire new migratory capacity. In addition, major histocompatibility complex (MHC) class II expression in the skin and intestine during GVHD renders these organs vulnerable to the cytotoxic activity of CD4+ effector T cells.5 The currently used National Institutes of Health criteria for acute GVHD grading6 are limited to the classical acute GVHD target organs. Future studies on the nonclassical target organs of acute GVHD and a consensus publication on the grading of nonclassical acute GVHD manifestations could help to extend these criteria. A more detailed grading of acute GVHD severity may help to better assess response to therapy and improve quality of life.
The phenomenon of “tissue tropism of acute GVHD” was previously explained by the presence of bacteria or bacterial components in the affected organs. Commensal bacteria reside on the skin and in the colon, and bacterial components drain via the portal vein system into the liver. Consistent with a role of the commensal bacteria at these anatomical sites, sequencing-based analysis of microbial flora of the human intestine and skin indicated that the commensal microbiome is frequently dysregulated and reduced in its diversity following allo-HCT and that this dysbiosis is connected to acute GVHD.7-10 Certain bacteria are associated with a better or worse outcome of patients developing acute GVHD. Blautia is connected to beneficial outcome,11 whereas Enterococci domination,12 which can be triggered by lactose consumption,13 is a risk for acute GVHD. Consistent with a pathogenic role of bacteria invading the barrier in the classical GVHD target organ, the elimination of bacteria that have penetrated by immunization against poly-N-acetylglucosamine reduced GVHD in mice.14 Also these invading bacteria elicit a local immune activation recruiting in particular innate immune cells as the first line of defense including neutrophils, macrophages, and monocytes.15-17 In addition to the bacterial components, sterile triggers of inflammation were shown to activate the immune system and enhance acute GVHD.18-20 These signals include, for example, the release of adenosine triphosphate from dying cells or uric acid, and they are also present at anatomical sides that lack commensal bacteria, such as nonclassical GVHD target organs.
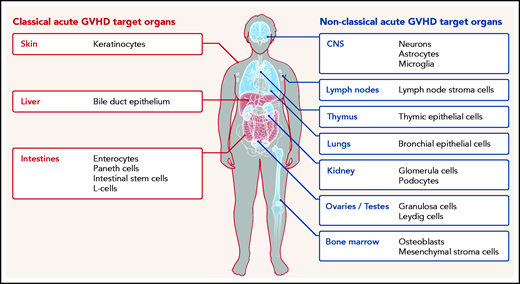
Furthermore, although commensal bacteria may enhance GVHD in the classical target organ, expression of alloantigens, which triggers alloreactive T-cell responses, is not restricted to the classical GVHD target organs but also present in relatively sterile organs such as the central nervous system (CNS), lungs, kidney, testes, ovaries, and bone marrow (Figure 1). In agreement with this, there is increasing evidence that the effects of acute GVHD are not limited to the 3 classical target organs but can also occur in other nonclassical organs. We performed a systematic literature review in the databases PubMed, PubMed Central, and Google Scholar using the terms “acute GVHD, target organs, allogeneic stem cell transplantation, GVHD manifestations” to identify evidence for acute GVHD affecting nonclassical GVHD target organs.
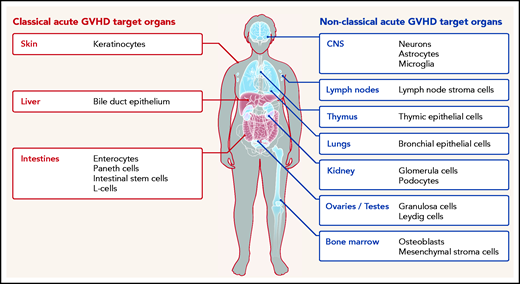
Classical (red) and nonclassical (blue) acute GVHD manifestations are shown. The typical GVHD target cell types that are primarily damaged by the alloreactive immune response are indicated. Additional cell types may be targets of aGVHD but further experimental evidence for their involvement is needed.
Classical (red) and nonclassical (blue) acute GVHD manifestations are shown. The typical GVHD target cell types that are primarily damaged by the alloreactive immune response are indicated. Additional cell types may be targets of aGVHD but further experimental evidence for their involvement is needed.
CNS
Mouse
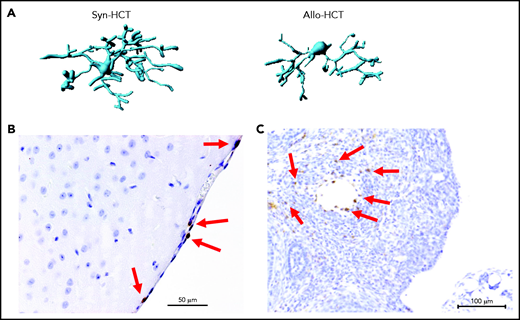
A nonclassical GVHD target organ that is considered to be sterile is the CNS. Mice transplanted without posttransplant immunosuppression display significant lassitude, behavioral depression, and impaired exploratory activity, spatial learning, and memory.21,22 Histologic analyses showed infiltration of effector memory T cells in the brain with the apoptotic cell death of neurons.21-23 CNS infiltration by CD8+ T cells was also demonstrated in a nonhuman primate model of GVHD.24 Importantly, pharmacologic GVHD prophylaxis inhibits donor T-cell infiltration to the CNS. Recently, the role of microglia for CNS inflammation during GVHD was described.25 GVHD caused activation of microglia cells, characterized by alterations in morphology (Figure 2A) and T-cell infiltration of the meninges (Figure 2B). Selective genetic ablation of transforming growth factor-β–activated kinase 1 (TAK1) or tumor necrosis factor (TNF) in microglia identified the TAK1/TNF/MHC-II axis as a central mediator of neuroinflammation.25 Additionally it was shown that indoleamine 2,3-dioxygenase is upregulated in microglial cells during GVHD in an interleukin 6 (IL-6)–dependent manner, and inhibition of IL-6 signaling attenuated neuroinflammation.22 Cell types damaged by acute GVHD in the CNS include neurons, astrocytes, and microglia.25
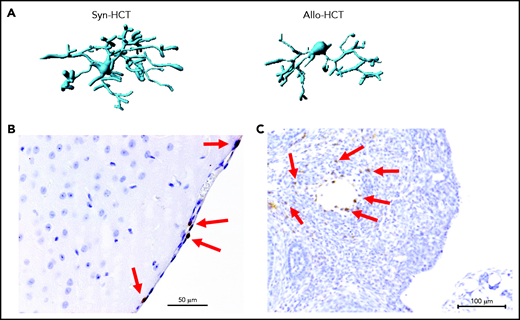
Microglia activation and T cell infiltration in nonclassical GVHD target organs. (A) Representative images showing Imaris-based (Bitplane) 3D reconstruction of Iba-1+ microglia cells from BALB/c mice on day 14 after syn-HCT or allo-HCT as indicated. (B) Histology of brain samples immunostained for CD3+ T cells (brown) from BALB/c mice on day 14 after allo-HCT. The arrows point toward CD3+ T cells. Scale bar, 50 μm. (C) Nonirradiated B6D2F1 mice were injected with 3 × 107 purified EGFP++ T cells and 5 × 107 wild-type B6 T cell–depleted bone marrow. The ovaries were analyzed on day +14. EGFP-positive (brown) donor T cells infiltrate in the ovarian follicles beyond the basement membrane. Scale bar, 100 μm.
Microglia activation and T cell infiltration in nonclassical GVHD target organs. (A) Representative images showing Imaris-based (Bitplane) 3D reconstruction of Iba-1+ microglia cells from BALB/c mice on day 14 after syn-HCT or allo-HCT as indicated. (B) Histology of brain samples immunostained for CD3+ T cells (brown) from BALB/c mice on day 14 after allo-HCT. The arrows point toward CD3+ T cells. Scale bar, 50 μm. (C) Nonirradiated B6D2F1 mice were injected with 3 × 107 purified EGFP++ T cells and 5 × 107 wild-type B6 T cell–depleted bone marrow. The ovaries were analyzed on day +14. EGFP-positive (brown) donor T cells infiltrate in the ovarian follicles beyond the basement membrane. Scale bar, 100 μm.
Human
The evaluation of the CNS in women undergoing female sex-mismatched bone marrow transplant revealed donor (Y-chromosome+)–derived cell infiltrates.26 Several studies (Table 1) have reported neurologic deficits and abnormal magnetic resonance imaging findings in patients developing acute or chronic GVHD.27 Most of these cases present with syndromes resembling multiple sclerosis, acute disseminated encephalomyelitis, transverse myelitis, and other nonspecific symptoms, in association with chronic GVHD.28 In patients with acute GVHD involving the CNS, upregulated TNF expression in microglia were observed in autopsy studies.25
Thymus and lymph nodes
Mouse
The thymus is exquisitely sensitive to damage by alloreactive T cells during acute GVHD, and its atrophy reflects contractions of the lymphatic and stromal compartment, leading to profound T-cell deficiency and T-cell receptor repertoire restriction.29-31 Reduced expression of tissue-restricted antigens in the thymus causes generation of autoreactive CD4+ T cells, which further promote GVHD.32 Functionally, donor-derived alloreactive T cells mediate thymic GVHD via Fas ligand and TNF-related apoptosis-inducing ligand–dependent mechanisms.33 Cell types damaged by acute GVHD in the thymus include thymic epithelial cells and thymus stroma cells, thereby impairing both positive and negative selection, allowing the emergence of autoreactive T cells in acute and chronic GVHD32,34 (Figure 1). Thymic epithelial cells are damaged by acute GVHD, and reduction of acute GVHD leads to improved thymic architecture.35 Lymph nodes are also targets of acute GVHD,36 and acute GVHD blocks peripheral tolerance of autoreactive T cells by impairing the display of peripheral tissue–restricted antigens in lymph nodes.36
Humans
Clinically, thymus GVHD is characterized as an impairment of thymic export of T cells; thus, it is difficult to identify in aged patients.37 The analysis of thymic T-cell receptor excision circles revealed that the thymus is affected by acute GVHD in humans but also that a certain degree of recovery is possible.38 In a prospective study of 52 children who underwent allo-HCT, the development of grade II to IV acute GVHD was the most important factor associated with low recent thymic emigrant counts.39
Lungs
Mouse
A causal relationship between alloreactivity and interstitial pneumonitis in mouse models of acute GVHD was reported by independent groups.40 Cell types damaged by acute GVHD in the lungs include bronchiolar epithelial cells (Figure 1). Histologic analyses revealed dense mononuclear cell infiltrates around both pulmonary vessels and bronchioles.41 Functional studies demonstrated that TNF-mediated endothelial apoptosis plays a key role in the development of experimental idiopathic pneumonia syndrome.42
Humans
Patients also experience lung GVHD, although it is difficult to distinguish between GVHD and organ toxicity by the conditioning regimen.43,44 Cooke et al45 reported that pulmonary acute GVHD occurred in 3% to 15% of all patients undergoing allo-HCT. A recent retrospective study in children identified acute GVHD as a significant risk for idiopathic pneumonia syndrome.46 Histologic analyses of the lung during acute GVHD showed interstitial and peribronchial lymphocytic infiltrate and acute bronchial epithelial degeneration.47,48 Endothelial cell damage has been implicated to contribute to the development of diffuse alveolar damage and transplant-associated microangiopathy.49,50
Kidney
Mouse/rat
Studies in a rat GVHD model suggest that the kidney is a target organ of acute GVHD.51 Acute GVHD of the kidney in this model was associated with increased levels of urinary 1-N-acetyl-β-d-glucosamine and signs of injury of the renal microvasculature and renal tubules.51 Studies in a murine model for acute GVHD revealed donor T-cell infiltration in the kidney, suggesting that acute GVHD can directly affect the kidney.52 A more recent study in mice reported that induction of acute GVHD caused glomerular injury and tubulointerstitial nephritis, which was mediated by activation of the complement system.53 Cell types damaged by acute GVHD in the kidney include glomerular cells (Figure 1).
Humans
In patients undergoing allo-HCT, acute kidney injury has been reported to manifest in approximately 70%.54,55 However, because multiple medications that are frequently used after allo-HCT, particularly calcineurin inhibitors, induce renal toxicity, it is difficult to quantify the contribution of immune-mediated kidney injury. Therefore, GVHD-associated kidney injury is most frequently suspected in the late phase after allo-HCT, when medications have been tapered in most patients. Most patients with suspected acute GVHD of the kidney exhibited nephrotic syndrome,56 and the clinical situation is often complicated by transplant-associated microangiopathy, in which microvascular endothelial injury occurs.57
Ovaries and testes
Mouse
A recent experimental study demonstrated that donor T-cell infiltration in close proximity to apoptotic granulosa cells in the ovarian follicles, resulting in impaired ovarian hormone production and maturation of ovarian follicles. Mating experiments revealed that female allo-HCT recipient mice deliver significantly fewer newborns than syngeneic HCT recipient mice, indicating conditioning-independent immunologic ovarian failure.58 However, it should be noted that the ovarian GVHD was observed in experiments not giving immunosuppressants as GVHD prophylaxis. When GVHD prophylaxis was given, the ovarian GVHD was suppressed, and fertility was restored. Although it is often difficult to distinguish conditioning-induced damage and GVHD-related organ damage, donor T-cell infiltration as an indicator of acute GVHD was detected in close proximity to apoptotic granulosa cells in the ovarian follicles (Figure 2C).58 This histologic finding was connected to reduced follicular hormone production and impaired maturation of ovarian follicles.
Additionally, the testis and its structures have been shown to be damaged by GVHD.59 The conditioning therapy will damage recipient tissues and thereby confound the magnitude of GVHD mediated tissue damage. To avoid artifacts by the conditioning, the authors used unconditioned F1 mice that received alloreactive T cells, leading to damage of intratesticular cells that was connected to the accumulation of donor alloreactive T cells. The authors also found a connection between low Leydig cell volume density and the peak of intratesticular donor T-cell infiltration and interferon-γ and TNF expression.59 Cell types damaged by acute GVHD in the testis include Leydig cells and granulosa cells (Figure 1).
Humans
Infertility associated with gonadal dysfunction is a serious late complication for long-term survivors of allo-HCT. Chemotherapy and irradiation cause premature gonadal failure independent of GVHD. It is likely that the contribution of acute GVHD to infertility after allo-HCT is rather small compared with these toxic effects. Nevertheless, results by the independent groups indicate impairment of sex hormone–producing granulosa cells and Leydig cells during acute GVHD. This correlates with the reported gonadal insufficiency and azoospermia after allo-HCT.60
Bone marrow
Mouse/rat
It was shown in mouse models of acute GVHD that osteoblasts and early B-cell lineages in the bone marrow (BM) are a target of GVHD.61 The authors could show that defective B lymphopoiesis was caused by the impaired ability of BM stroma to support the hematopoiesis.61 The BM microenvironment is a key player in regulation and maintenance of hematopoiesis, and its dysregulation by oncogenic mutations heavily affects the functional output.62,63 Nonhematopoietic BM nice cells, including mesenchymal stem and progenitor cells, endothelial cells, osteoblasts, and CXCL12-abundant reticular cells, are of recipient origin and therefore can be recognized as foreign by the donor T cells. Allogeneic antiosteoblast reactions can affect hematopoiesis as it was shown that osteoblasts are required for maintaining hematopoietic stem cells self-renewal and differentiation.64 GVHD-affected BM was unable to reconstitute the hematopoietic cells in secondary recipients, whereas the treatment with anti-CD4 monoclonal antibody improved B lymphopoiesis.61 Cell types damaged by acute GVHD in the BM also include osteoblasts and mesenchymal stroma cells (MSCs; Figure 1).
Humans
Consistent with the findings in mice, clinical and experimental data have shown that immune reconstitution is impaired by GVHD.65 The analysis of BM isolated from allo-HCT patients revealed that donor T cells were present in the BM and that osteoblast numbers declined on GVHD development.66 Functionally, the occurrence of BM GVHD was connected to delayed B-cell reconstitution and impaired antibody responses in patients.66 MSCs isolated from patients with acute GVHD displayed impairment of osteogenic and adipogenic differential capacity, colony formation capacity, and expression of self renewal–related genes.67 Furthermore, the hematopoiesis-supporting capacity of MSCs declined during acute GVHD in a TNF-dependent fashion.67
Discussion
Emerging evidence derived from studies in mouse models of acute GVHD indicate that donor T cells could attack nonclassical target tissues. However, it should be noted that in most experimental studies, pharmacologic GVHD prophylaxis was not given. When immunosuppression such as calcineurin inhibitors, mycophenolic acid, posttransplant cyclophosphamide (PTCY), or anti-thymocyte globuline are given, nonclassical GVHD is less pronounced and may remain on a level that is hard to detect clinically. Nonetheless, these observations are highly relevant in allo-HCT, because evidence for injury in nonclassical GVHD target organs also comes from studies in humans. The CNS was shown by several investigators to be affected by acute GVHD in rodent models and humans.21,25 Therefore, future studies that will address the neurologic functions more closely when GVHD evolves could help shape CNS–GVHD response criteria. CNS–GVHD may be more frequent than expected because a recent study showed a high frequency of noninfectious neurologic complications after allo-HCT.68 The authors found in a multivariable analysis that the development of acute GVHD grade II to IV and extensive chronic GVHD were independently associated with increased risk of neurologic complications.68 However, multiple causes for neurologic complications after allo-HCT exist, and it is still unclear how much acute GVHD contributes. A better understanding of the involvement of the thymus and lymph nodes in acute GVHD could help to develop novel strategies to reduce immunodeficiency after allo-HCT (eg, using regenerative approaches with keratinocyte growth factor69 or IL-770). Such tissue regenerative approaches may not only help in nonclassical target organs but also in classical acute GVHD target organs like the intestinal tract by using R-spondin71,72 or glucagone like peptide-273 to promote expansion of Paneth cells and intestinal stem cells. Lung GVHD is a major complication after allo-HCT that is connected to high mortality. Reducing this nonclassical manifestation of acute GVHD could help to decrease the incidence of chronic lung GVHD because acute GVHD is a major risk factor for chronic GVHD. This is particularly important because pulmonary chronic GVHD is hard to treat, even in the era of novel second-line GVHD therapies including ruxolitinib.74-76 Combination of ruxolitinib with extracorporeal photopheresis may have some activity on chronic GVHD of the lung77; however, prospective trials are needed to confirm retrospective analyses. Acute GVHD of the testes and ovaries was neglected for a long time, and reduced fertility was thought to be only caused by conditioning-related toxicity. However, murine studies could show that donor T cells damage the granulosa cells in the ovarian follicles, resulting in impaired ovarian hormone production and maturation of ovarian follicles58 and the testis.59 The diagnosis of acute GVHD of the kidney is difficult because of multiple confounding factors (eg, calcineurin inhibitors; certain anti-cytomegalovirus medications such as ganciclovir, foscavir, or cidofovir; antibiotics such as tobramycin or vancomycin; and antifungal agents such as amphothericin B can cause renal damage).
The reports on acute GVHD affecting the BM and thereby hematopoietic function are clinically relevant because poor graft function and graft failure are major clinical challenges. The observation that donor T cells infiltrate in the BM where they damage different types of stroma cells supports the use of immunosuppression in patients with poor engraftment and evidence for acute GVHD.66 Besides these clinical considerations, a major question is why acute GVHD was for a long time regarded as tissue specific for liver, intestine, and skin. Besides the theory of commensal bacteria being present in the intestines, skin, and liver portal vein system, an increased release of cytokines and chemokines in these tissues could contribute to tissue tropism. Cytokine-release syndrome (CRS), also referred as cytokine storm, was described as a key feature of acute GVHD by Ferrara and Antin in 1992.78 In haploidentical hematopoietic stem cell transplantation using PTCY, usually CSP or tacrolismus is not given before PTCY,79 leading to the development of CRS or so-called haploimmune fever.80 Proinflammatory cytokines are key effector molecules to cause acute GVHD in mice.81 Multiple clinical trials have tested cytokine inhibition for GVHD treatment, and a recent randomized phase 3 study testing the Janus kinase 1/2 inhibitor ruxolitinib that inhibits downstream signaling of multiple cytokine receptors showed a higher overall response rate in patients treated with this approach for corticosteroid-refractory acute GVHD compared with best available therapy.74 In recent years, an evolution of the therapeutic landscape in GVHD has taken place, with novel inhibitors targeting kinases such as rho-associated, coiled-coil containing protein kinase and Janus kinase, demethylating agents, transfer of immunomodulatory cells, and transfer of microbiota. Currently, only limited data on the impact of these novel approaches on nonclassical acute GVHD manifestations are available. A major future task will be to implement the nonclassical manifestations of acute GVHD into the National Institutes of Health criteria for acute GVHD grading. It is conceivable that the cytokine storm preceding acute GVHD has similarities with CRS caused by chimeric antigen receptor T-cell therapy , which induces multiorgan dysfunction, involving the brain, lung, and kidney.
To improve the quality of life and survival of our patients on allo-HCT, it will be important to detect and treat acute GVHD of nonclassical target organs. To identify CNS GVHD early, it may be helpful to perform neurologic testing (eg, minimental status test) at multiple time points, followed by CNS imaging if the mental status worsens without other clear reasons. Regular lung function testing at defined time points (eg, days 30, 60, 100, and 160 after allo-HCT) can help to detect pulmonary impairment early and to increase immunosuppression after excluding infectious causes.
BM function and kidney function are regularly monitored by blood analysis in current practice. However, it is often challenging to differentiate medication-induced toxicity from real acute GVHD. Hematopoiesis can be affected by multiple medications (eg, valgancyclovir) that are frequently used after allo-HCT. Also, poor graft function independent from GVHD can cause cytopenias. Besides these factors, acute GVHD should be considered and treated.
As the medical technology progresses, nonclassical target organ injury in acute GVHD may become more apparent. Results in animal studies deserve further clinical scrutiny, but they promise to open a new avenue to reduce previously less recognized tissue injury that was masked by conditioning toxicity. Overall, there is a need to develop strategies to detect the nonclassical acute GVHD manifestations earlier and to develop a consensus for their diagnosis and treatment.
Acknowledgments
The authors thank Michal Rössler for preparing Figure 1.
R.Z. was supported by the Deutsche Forschungsgemeinschaft for the SFB 1479 OncoEscape P01, Project 441891347 (grants SFB1160, ZE 872/4-2, and TRR167); Deutsche Krebshilfe (grant 70113473); and Jose-Carreras Leukemia Foundation grant DJCLS 01R/2019.
Authorship
Contribution: T.T. and R.Z. wrote the paper.
Conflict-of-interest disclosure: R.Z. received honoraria from Incyte, Novartis, and Mallinckrodt. T.T. received grants from Kyowa Kirin, Chugai, Sanofi, Astellas, Teijin Pharma, Fuji Pharma, and Nippon Shinyaku; personal fees from Novartis, Merck, Kyowa Kirin, Takeda, Pfizer, and Bristol-Myers Squibb; and nonfinancial support from Janssen and Novartis.
Correspondence: Takanori Teshima, Department of Hematology, Hokkaido University Faculty of Medicine, N15 W7, Kita-Ku, Sapporo 060-8638, Japan; e-mail: teshima@med.hokudai.ac.jp.