TO THE EDITOR:
For the past 75 years, debate has focused on 2 primary mechanisms for platelet generation: cytoplasmic fragmentation and proplatelet production. The cytoplasmic fragmentation model is based on high-magnification images visualizing the internal ultrastructure of megakaryocytes.1 These images show that megakaryocytes contain abundant membranes that divide the cell into platelet territories, a term indicating a role in partitioning off assembling platelets formed by a fracturing of the megakaryocyte cytoplasm. In contrast, the proplatelet model proposes that megakaryocytes generate platelets by remodeling their cytoplasm into long beaded processes that function as the assembly lines of platelet production (Figure 1A).2 Proplatelets are thought to function as intermediate structures in platelet production, and platelets generated from proplatelets manifest many properties of blood platelets.3 Intravital microscopy visualizing megakaryocytes forming extensions in the bone marrow of living mice also supports a physiological role for proplatelets. According the proplatelet model, the demarcation system provides a reservoir of platelet membranes to cover growing proplatelet processes.4 In 2007, Junt et al5 observed living megakaryocytes extending proplatelets into the vascular lumen and demonstrated how flowing blood sheared these processes off into the circulation. According to 2-photon and light-sheet microscopy data from Geue et al,6 Münzer et al,7 and Stegner et al,8 ∼1620 megakaryocytes per mm3 of bone marrow in mice generate proplatelets at steady state.
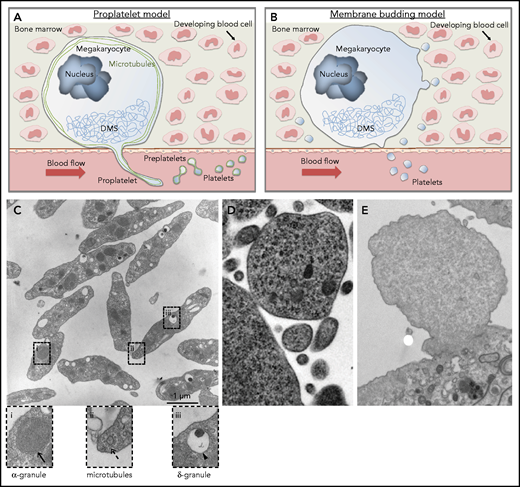
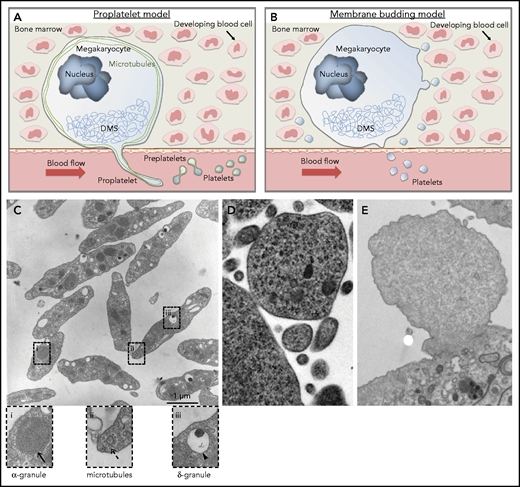
Schematic of current concepts of platelet production showing mature megakaryocytes localized close to bone marrow sinusoids. (A) Proplatelet model. Megakaryocytes polarize their demarcation membrane system (DMS) as prerequisite for directed release of long cytoplasmic protrusions called proplatelets, which are driven by reorganization of microtubules (shown in green) into the sinusoidal lumen. Platelet intermediates (preplatelets) and platelets are shed into the circulation by blood shear forces. The terminal stage of platelet production occurs in the bloodstream. (B) Membrane budding model. Continuous release of final platelets directly from the megakaryocyte via membrane budding. (C) Thin-section electron micrograph showing the hallmark features of blood platelets. Resting platelets have a discoid shape with clear α-granules (i), microtubules (ii), and dense granules (iii). (D) Electron micrograph showing a megakaryocyte membrane bud. Reproduced from Potts et al9 (© 2020 Potts et al) under a CC BY-NC-SA 4.0 license. (E) Electron micrograph showing a bleb from a cultured mouse megakaryocyte that lacks the characteristic features of an assembling platelet. Scale bar, 1 μm. Cells were fixed, processed, and imaged as previously described.10
Schematic of current concepts of platelet production showing mature megakaryocytes localized close to bone marrow sinusoids. (A) Proplatelet model. Megakaryocytes polarize their demarcation membrane system (DMS) as prerequisite for directed release of long cytoplasmic protrusions called proplatelets, which are driven by reorganization of microtubules (shown in green) into the sinusoidal lumen. Platelet intermediates (preplatelets) and platelets are shed into the circulation by blood shear forces. The terminal stage of platelet production occurs in the bloodstream. (B) Membrane budding model. Continuous release of final platelets directly from the megakaryocyte via membrane budding. (C) Thin-section electron micrograph showing the hallmark features of blood platelets. Resting platelets have a discoid shape with clear α-granules (i), microtubules (ii), and dense granules (iii). (D) Electron micrograph showing a megakaryocyte membrane bud. Reproduced from Potts et al9 (© 2020 Potts et al) under a CC BY-NC-SA 4.0 license. (E) Electron micrograph showing a bleb from a cultured mouse megakaryocyte that lacks the characteristic features of an assembling platelet. Scale bar, 1 μm. Cells were fixed, processed, and imaged as previously described.10
In May 2020, Potts et al9 published an article in Journal of Experimental Medicine in which they offer a completely different hypothesis for platelet production, proposing that membrane budding is the major mechanism for platelet production (Figure 1B). Using 3- and 4-dimensional imaging methods that enable megakaryocyte behavior to be studied in its native environment, the authors suggest that membrane budding is a common event that results in the substantial release of platelets directly into the peripheral circulation during both fetal and adult life. They show that buds derive from the surface of megakaryocytes and suggest that these buds are directly released as platelets. Using NF-E2 knockout mice, which exhibit severe thrombocytopenia, the authors suggest that a block in megakaryocyte budding explains the low platelet count observed in these mice.
The findings of Potts et al9 are provocative; however, whether the budding of the megakaryocyte surface generates bona fide platelets remains uncertain. We previously noted that microvesicles can be shed from maturing megakaryocytes (MKMVs).10 This work showed that cultured megakaryocytes produced MKMVs that were smaller and less regular than platelets and lacked standard platelet organelles such as granules and mitochondria. However, these earlier studies did not include imaging of MKMV formation in vivo. In contrast, Potts et al included in vivo studies, but not high-magnification imaging of megakaryocytes in bone marrow. They were therefore unable to directly address the question of whether the structures they studied contained a normal allotment of organelles. To fill this gap in our knowledge and assess the nature of the buds emanating from megakaryocytes in vivo, we compared micrographs of bona fide platelets with the structures observed by Potts et al and evaluated high-magnification images of megakaryocytes in bone marrow.
Assessment of the images in Potts et al9 leads us to question whether the objects they describe are actually platelets. There are clear morphological and ultrastructural differences between platelets and MKMVs (Figure 1C-E). If membrane budding were a major mechanism of platelet production, one would expect the budding megakaryocyte cytoplasm to exhibit characteristics of a resting platelet and possess an array of intracellular components. Blood platelets display several distinguishing features, including a discoid shape, microtubule coils, an open canalicular system, and distinct organelles (Figure 1C). In the initial figure of Potts et al, there is very little evidence indicating that budding megakaryocytes have the ultrastructural features of blood platelets, because electron micrographs do not show clear evidence of microtubule coils, granules, or an open canalicular system (Figure 1D). In fact, this cell fragment looks more like a microvesicle from a cultured blebbing megakaryocyte (Figure 1E). In addition to functioning as the band aids of the blood, platelets also serve as a major transport and delivery system. To accomplish this, platelets contain major secretory granules, including α-granules and dense granules, which are clearly visible by electron microscopy in blood platelets (Figure 1C).
To better understand the in vivo significance of the blebbing phenomenon, we evaluated megakaryocytes in their native bone marrow environment at high magnification using transmission electron microscopy (Figure 2). Strikingly, these images demonstrated a central area replete with vesicles and other organelles and a rim of cytoplasm around the central area devoid of organelles and from which the blebs could be seen pinching off (Figure 2A; supplemental Figure 1). These images suggest that this rim of cytoplasm gives rise to MKMVs (Figure 2B), which are reminescent of the budding vesicular structures observed by Potts et al.9 Megakaryocytes situated in the bone marrow were frequently observed extending blebs toward blood vessels (Figure 2C-D). However, these blebs appeared to lack microtubule coils, granules, and an open canalicular system, hallmark features of assembling blood platelets. To assess the nature of megakaryocyte blebs more comprehensively, we performed a quantitative comparison of the α-granule content of 32 megakaryocyte blebs and 26 mature platelets. To correct for sample error inherent in analysis of transmission electron microscope images and for differences in size between megakaryocyte blebs and platelets, α-granule density (ie, α-granules per μm2) was calculated for blebs and platelets (Figure 2E). Areas and α-granule numbers were quantified from images of bone marrow megakaryocytes and mature circulating platelets (supplemental Figure 2). This analysis showed that most megakaryocyte blebs had no α-granules, and the overall α-granule density of megakaryocyte blebs was <0.09 α-granules per μm2, whereas mature platelets had an α-granule density of 2.8 α-granules per μm2. These observations support a model wherein the cytoplasmic rim surrounding the organelle-replete central area of the megakaryocyte actively contributes to the formation of blebs that are mostly devoid of α-granules and other organelles observed in mature platelets.10
Thin-section electron micrographs showing blebbing megakaryocytes (MKs) in the bone marrow. (A) Bone marrow MKs demonstrate a central organelle-rich area (thick arrows) surrounded by an organelle-deplete cyoplasmic rim (thin arrows). (B) MK blebs lack the characteristic contents and features of an assembling platelet. (C-D) Blebbing MKs are frequently observed in the marrow adjacent to blood vessels. Scale bars, 2 μm. (E) Comparison of α-granule content in MK blebs vs mature platelets. TEM images of MK blebs (n = 32) and mature platelets (n = 26; supplemental Figure 2) were analyzed for α-granule number and total area; α-granule density (α-granule per μm2) is shown as mean ± standard deviation. EC, endothelial cell; RBC, red blood cell.
Thin-section electron micrographs showing blebbing megakaryocytes (MKs) in the bone marrow. (A) Bone marrow MKs demonstrate a central organelle-rich area (thick arrows) surrounded by an organelle-deplete cyoplasmic rim (thin arrows). (B) MK blebs lack the characteristic contents and features of an assembling platelet. (C-D) Blebbing MKs are frequently observed in the marrow adjacent to blood vessels. Scale bars, 2 μm. (E) Comparison of α-granule content in MK blebs vs mature platelets. TEM images of MK blebs (n = 32) and mature platelets (n = 26; supplemental Figure 2) were analyzed for α-granule number and total area; α-granule density (α-granule per μm2) is shown as mean ± standard deviation. EC, endothelial cell; RBC, red blood cell.
The megakaryocyte is remarkable in its ability to produce platelets that are relatively uniform in size and content. Any theory of platelet production by megakaryocytes must provide a mechanism by which homogenous platelet populations are generated. Production of platelets via proplatelet formation involves the elongation of slender tubules of uniform diameter that enable generation of uniform platelets.11 In contrast to platelets, platelet microvesicles vary significantly in size, much like the blebs on maturing megakaryocytes. A thorough model of platelet production needs to explain how each platelet receives the proper allotment of essential organelles/mitochondria, dense granules, α-granules, and so on. Cytoskeletal transport pathways provide a mechanistic explanation for the loading of assembling platelets with organelles and granules.12 An indiscriminate budding mechanism fails to address this issue. In contrast, MKMVs vary substantially in size and do not possess the full allotment of organelles that characterize platelets.
In summary, we propose that the studies described by Potts et al9 do not demonstrate a novel pathway for generating mature platelets, but rather describe the generation of an alternative megakaryocyte-derived product. It is possible that these blebbing structures are related to circulating MKMVs, which derive from megakaryocytes and do not possess a normal repertoire of organelles.10 MKMVs have been shown to have several important functions in megakaryopoiesis, thrombosis, and cell-cell communication.13-15 Our high-resolution electron microscopy of megakaryocytes in the bone marrow environment demonstrates that MKMV-like structures form in vivo from a rim of cytoplasm that blebs off into vesicular structures in close apposition to the bone marrow vasculature. Whether the MKMVs that we observed in bone marrow are the same as those we previously described in the circulation10 and whether they are the same structures described by Potts et al will require additional studies. Nonetheless, our evaluation of bone marrow megakaryocytes at high resolution raises substantial doubt about whether the structures derived from megakaryocyte budding described by Potts et al are bona fide platelets. The argument that megakaryocyte blebbing is not a physiological mechanism for generating genuine circulating platelets derives not from a single observation, but rather from a confluence of evidence. Lack of morphological platelet features, bleb uniformity, demonstration of bleb disconnection, and integrated regulation in response to physiological cues challenge the contention that mature circulating platelets form from megakaryocyte blebs. These concerns underscore the importance of rigorous assessment of platelet morphology and careful consideration of MKMV production in proposing alternative models of platelet biogenesis.
Acknowledgments
This work was supported in part by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (R01HL68130 [J.E.I.] and R35HL135775 [R.F.]), and the German Research Foundation (project 374031971–CRC/TR 240 [M.B. and B.N.]).
Authorship
Contribution: J.E.I. and R.F. conceived the study. J.E.I., G.M.-S., R.F., and M.B. performed studies. J.E.I., M.B., C.G., B.N., and R.F. wrote and edited the manuscript. All authors read and approved the final manuscript.
Conflict-of-interest disclosure: J.E.I. is a cofounder of and has financial interest in PlateletBio, a biotechnology company that aims to produce donor-independent platelet-like cells. R.F. is a founder of and has financial interest in PlateletDiagnostics. The interests of R.F. are reviewed and managed by the Beth Israel Deaconess Medical Center Office of Compliance and Business Conduct. The remaining authors declare no competing financial interests.
Correspondence: Robert Flaumenhaft, Division of Hemostasis and Thrombosis, Department of Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215; e-mail: rflaumen@bidmc.harvard.edu.
The online version of this article contains a data supplement.