Key Points
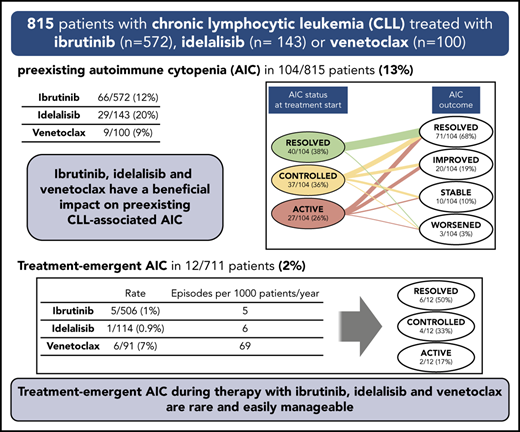
Ibrutinib, idelalisib, and venetoclax have a beneficial impact on preexisting AICs associated with CLL.
Treatment-emergent AICs are more frequent, though easily manageable, during treatment with venetoclax than with ibrutinib or idelalisib.
Abstract
Autoimmune cytopenias (AICs) affect 5% to 9% of patients with chronic lymphocytic leukemia (CLL). Targeted drugs—ibrutinib, idelalisib, and venetoclax—have a prominent role in the treatment of CLL, but their impact on CLL-associated AICs is largely unknown. In this study, we evaluated the characteristics and outcome of preexisting AICs and described the incidence, quality, and management of treatment-emergent AICs during therapy with targeted drugs in patients with CLL. We collected data from 572 patients treated with ibrutinib (9% in combination with an anti-CD20 monoclonal antibody), 143 treated with idelalisib-rituximab, and 100 treated with venetoclax (12% in combination with an anti-CD20 monoclonal antibody). A history of preexisting AICs was reported in 104 (13%) of 815 patients. Interestingly, 80% of patients whose AICs had not resolved when treatment with a targeted drug was started experienced an improvement or a resolution during therapy. Treatment-emergent AICs occurred in 1% of patients during ibrutinib therapy, in 0.9% during idelalisib therapy, and in 7% during venetoclax therapy, with an estimated incidence rate of 5, 6, and 69 episodes per 1000 patients per year of exposure in the 3 treatment groups, respectively. The vast majority of patients who developed treatment-emergent AICs had unfavorable biological features such as an unmutated IGHV and a del(17p) and/or TP53 mutation. Notably, despite AICs, 83% of patients were able to continue the targeted drug, in some cases in combination with additional immunosuppressive agents. Overall, treatment with ibrutinib, idelalisib, or venetoclax seems to have a beneficial impact on CLL-associated AICs, inducing an improvement or even a resolution of preexisting AICs in most cases and eliciting treatment-emergent AICs in a negligible portion of patients.
Introduction
Autoimmune cytopenias (AICs) are a frequent complication in chronic lymphocytic leukemia (CLL), affecting 5% to 9% of patients.1-7 AICs can present as autoimmune hemolytic anemia (AIHA), immune thrombocytopenia (ITP) or, more rarely, as pure red blood cell aplasia or autoimmune granulocytopenia. Treatment of CLL-associated AICs is generally primarily directed toward the autoimmune phenomenon, whereas patients with refractory disease or patients with additional signs of disease progression usually receive CLL-specific therapy.8
In the last few years, targeted drugs such as ibrutinib, idelalisib, and venetoclax have entered the therapeutic armamentarium for patients with CLL, showing excellent results in terms of efficacy.9 The activity of these compounds on CLL-associated AICs is largely unknown because patients with active AICs have been excluded from the pivotal clinical trials and because of the paucity of studies aimed at investigating the role of these novel signal inhibitors in this setting. In addition, there are no guidelines to direct the management of patients who develop AICs during treatment with targeted drugs.
The BTK inhibitor ibrutinib may have a role in controlling autoimmunity, based on its demonstrated activity on the T-cell and monocyte/macrophage compartments.10-12 However, the initially reported clinical effects were controversial, with cases of refractory CLL-associated AIHA successfully treated with ibrutinib,13-16 but also acute flares of AIC occurring after ibrutinib was initiated.17,18 In larger series, data from an ad hoc analysis of the phase 3 RESONATE trial and from retrospective evaluations of patients treated in clinical studies and in real-world practice show enhanced control of preexisting AICs and a low rate of treatment-emergent AICs during treatment with ibrutinib.19-22
Idelalisib, a PI3K inhibitor, is associated with a significant incidence of nonhematologic autoimmune complications, including autoimmune hepatitis, colitis, and pneumonitis,23,24 which led some clinicians to avoid idelalisib, if possible, for treatment of CLL in the presence of AICs.25 Data on the use of idelalisib for managing AICs are therefore very limited, but a 95% response rate for the autoimmune phenomena was reported in a French series of 19 patients with CLL-associated AICs who received idelalisib and rituximab.22 To our knowledge, no study has specifically investigated the frequency of treatment-emergent AICs during idelalisib-based regimens.
Information regarding the efficacy of the Bcl-2 inhibitor venetoclax for treating patients with CLL-associated AICs is even more scarce, and at this point is limited to case reports.26,27 Interestingly, although they have not been systematically evaluated in specific studies, treatment-emergent AICs were reported among adverse events (AEs) in clinical trials that investigated the efficacy of venetoclax, alone or in combination with rituximab.28-30
The objective of this study was to perform a retrospective analysis of a large multicenter cohort of patients with CLL treated with ibrutinib, idelalisib, or venetoclax with the aims of specifically evaluating the characteristics and outcome of preexisting AICs and systematically describing the incidence, quality, and management of treatment-emergent AICs.
Methods
We retrospectively evaluated a multicenter cohort of 815 consecutive patients with CLL treated with ibrutinib, idelalisib, or venetoclax in 15 Italian centers (supplemental Table 1, available on the Blood Web site). The study was conducted in accordance with the Declaration of Helsinki and was approval by the local ethics committees. Investigators collected data regarding demographics, disease characteristics, AIC history and management, and treatment with targeted drugs. AICs were diagnosed and managed according to the treating physician. However, precise definitions of AICs were shared among investigators (supplemental Table 2) to categorize preexisting and treatment-emergent AICs as well as their status and outcome (Table 1). Preexisting AICs were defined as episodes occurring before the start of targeted drug treatment, regardless of their status at initiation of treatment. Treatment-emergent AICs were defined as episodes occurring at any time during treatment with targeted drugs in the absence of a known preexisting AIC.
The response of CLL to treatment was defined on the basis of the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines.31 Data regarding best response achieved at any time during treatment were collected, but because of study design, the time for response assessments was not standardized. Patients fulfilling all criteria for complete response (CR) but who did not undergo a restaging bone marrow biopsy were defined as having a partial response (PR). AEs were defined according to the Common Terminology Criteria for Averse Events (version 4.0), except for hematologic toxicities, which were graded in accordance with the iwCLL grading scale.31
Descriptive statistics were used to summarize patients’ characteristics. Features of patients with or without preexisting AICs and with or without treatment-emergent AICs were compared using Fisher’s exact test. Progression-free survival (PFS) was defined as time from start of treatment with the targeted drug to disease progression or death from any cause. Overall survival (OS) was defined as time from start of treatment with the targeted drug to death. PFS and OS were estimated by using the Kaplan-Meier method, and differences between groups were evaluated with the log-rank test. The patient-time incidence rate of treatment-emergent AICs in each treatment group was estimated by dividing the number of AIC episodes by the total number of years the patients were at risk (ie, treatment duration). Rates in treatment groups were compared using Fisher’s exact test.
Statistical analyses were performed using SPSS Statistics software version 22.0 for Windows (IBM Corp, Armonk, NY), GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA), and OpenEpi version 3.01 (www.OpenEpi.com). A value of P < .05 was considered significant.
Results
Characteristics of the cohort
A total of 815 patients were identified, including 572 treated with ibrutinib, 143 treated with idelalisib, and 100 treated with venetoclax. Patients’ characteristics at the time of starting treatment with the targeted drug are provided in Table 2. The majority of patients had previously received a CLL-directed therapy (72% in the ibrutinib group, 91% in the idelalisib group, and 88% in the venetoclax group). A relevant proportion of patients had unfavorable biological prognostic factors, including unmutated immunoglobulin heavy chain variable gene (IGHV) status in 71% (72% in the ibrutinib group, 69% in the idelalisib group, and 71% in the venetoclax group), del(11q) in 15% (14% in the ibrutinib group, 18% in the idelalisib group, and 16% in the venetoclax group), del(17p) in 38% (39% in the ibrutinib group, 40% in the idelalisib group, and 33% in the venetoclax group), and TP53 mutation in 33% (33% in the ibrutinib group, 30% in the idelalisib group, and 43% in the venetoclax group).
Ibrutinib was given as a single agent in the majority of patients (91%), whereas 50 (9%) of 572 patients received ibrutinib in combination with an anti-CD20 monoclonal antibody (ibrutinib plus rituximab, n = 39; ibrutinib plus ofatumumab, n = 9; ibrutinib plus obinutuzumab, n = 2). Idelalisib was given in association with rituximab in all patients. Eighty-eight percent of patients treated with venetoclax received the drug as monotherapy, whereas in 12 (12%) of 100 patients, it was given in association with an anti-CD20 monoclonal antibody (venetoclax plus rituximab, n = 11; venetoclax plus obinutuzumab, n = 1).
The median follow-up from the start of the targeted drug was 31 months for the ibrutinib group (range, 0-78 months), 43 months for the idelalisib group (range, 0-102 months), and 14 months for the venetoclax group (range, 1-70 months). The median duration of treatment was 23 months for the ibrutinib group (range, 0-74 months), 14 months for the idelalisib group (range, 0-98 months), and 11 months for the venetoclax group (range, 1-70 months), with 66%, 26%, and 70% of patients, respectively, still receiving treatment at the last follow-up in the 3 cohorts. In the ibrutinib cohort, the overall response rate (ORR) was 87%, with 7% CRs and 80% PRs (data available for 560 patients). In idelalisib-treated patients, the ORR was 84%, with only 1 patient achieving a CR (data available for 138 patients). In the venetoclax cohort, the ORR was 78%, with a CR rate of 11% and a PR rate of 67% (data available for 99 patients).
Patients treated with ibrutinib had a median PFS of 50 months, with a 24-month PFS of 79%, and OS rates of 88% at 24 months and 78% at 36 months (median OS, not reached). In the idelalisib group, the median PFS was 25 months (24-month PFS, 57%) and the median OS was 56 months (OS rates, 77% at 24 months and 65% at 36 months). Venetoclax-treated patients had PFS rates of 80% at 12 months and 73% at 24 months and OS rates of 86% at 12 months and 76% at 24 months (median PFS and OS, not reached).
Preexisting AICs
A preexisting AIC was reported in 104 (13%) of 815 patients: 66 (12%) of 572 ibrutinib-treated patients, 29 (20%) of 143 idelalisib-treated patients, and 9 (9%) of 100 venetoclax-treated patients (Table 3). The most frequent AIC manifestation was AIHA followed by ITP in all treatment groups. Nearly all patients with a preexisting AIC had received a previous AIC-directed treatment before starting treatment with a targeted drug (95% of ibrutinib-treated patients and 100% of idelalisib- and venetoclax-treated patients). Previous AIC-directed treatments were diverse: treatment with steroids only was given to 49% of patients in the ibrutinib group, 42% in the idelalisib group, and 78% in the venetoclax group, whereas other patients received drugs of multiple classes sequentially or in combination (Table 3).
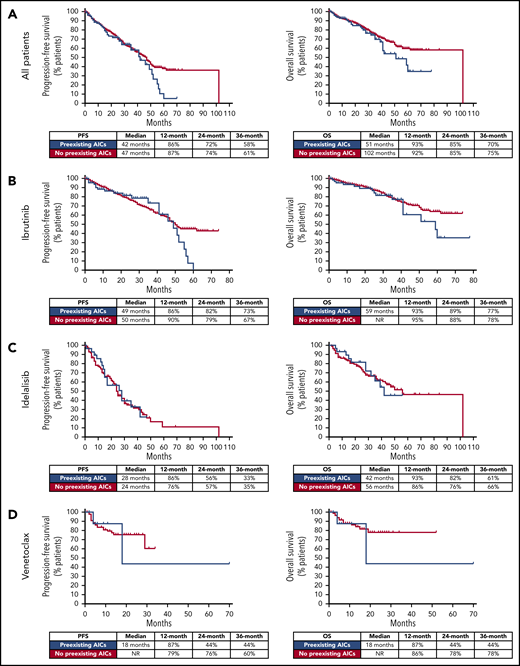
The main clinical and biological data at the start of treatment in patients with or without preexisting AICs were evaluated (supplemental Tables 3 and 4). When patients who received ibrutinib, idelalisib, or venetoclax were analyzed together, the presence of a preexisting AIC correlated significantly with an adverse fluorescence in situ hybridization (FISH) result (65% of patients had del(17p) and/or del(11q) vs 52% in patients with no preexisting AICs; P = .022), severe hypogammaglobulinemia (17% of patients had immunoglobulin G (IgG) values <300 mg/dL vs 9% in patients with no preexisting AICs; P = .033), and pretreatment status (39% of the patients had received >2 previous lines of therapy vs 26% in patients with no preexisting AICs; P = .007). By contrast, no correlation was observed between the presence of preexisting AICs and the IGHV mutational status. When treatment cohorts were analyzed separately, the presence of a preexisting AIC maintained a statistically significant association with severe hypogammaglobulinemia (19% of patients had IgG values <300 mg/dL vs 8% in patients with no preexisting AICs; P = .046) and pretreatment status (33% of patients had received >2 previous lines of therapy vs 20% in patients with no preexisting AICs; P = .025) in the ibrutinib group, and with adverse FISH results (89% of patients had del(17p) and/or del(11q) vs 45% in patients with no preexisting AICs; P = .015) in the venetoclax group. Moreover, in the entire cohort, patients with preexisting AICs had a significantly higher prevalence of grade 3 AEs during treatment with a targeted drug compared with patients with no preexisting AICs (52% vs 37%; P = .006). Of note, in our analysis, patients with or without preexisting AICs did not significantly differ in terms of response to targeted agents (data not shown) or in terms of PFS and OS (Figure 1).
PFS and OS in patients with and without preexisting AICs. In the entire cohort (A), and in the ibrutinib (B), idelalisib (C), and venetoclax (D) groups, PFS and OS were not significantly different in patients with and without preexisting AICs.
PFS and OS in patients with and without preexisting AICs. In the entire cohort (A), and in the ibrutinib (B), idelalisib (C), and venetoclax (D) groups, PFS and OS were not significantly different in patients with and without preexisting AICs.
Evolution of preexisting AICs during treatment with targeted drugs
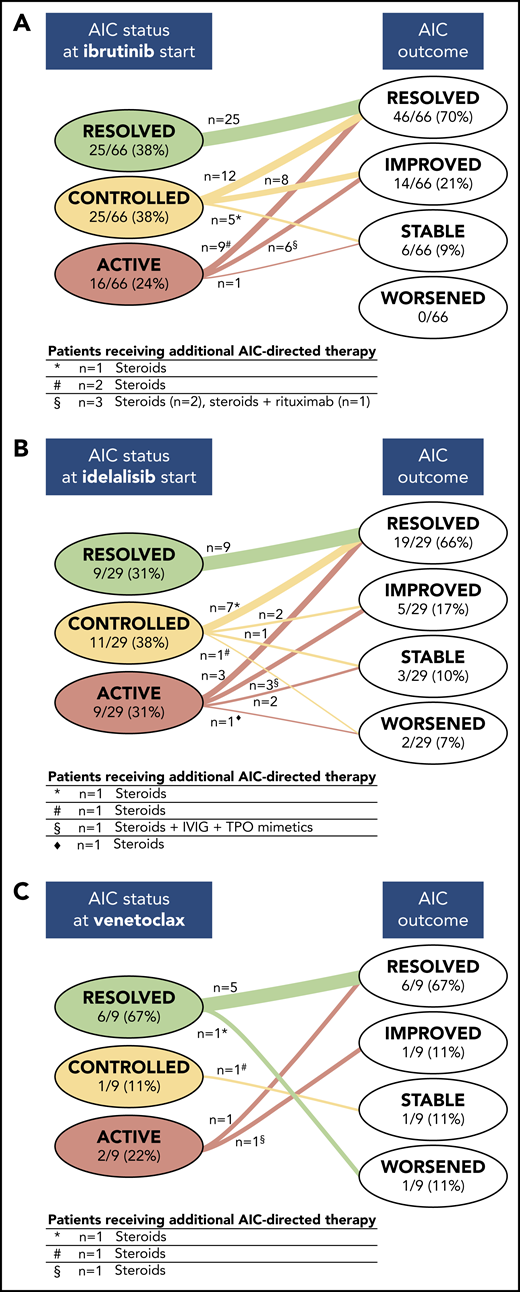
The status of preexisting AICs at the start of treatment with a targeted drug and their evolution during treatment is depicted in Figure 2. When treatment with ibrutinib was started, the preexisting AIC was considered active in 16 (24%) of 66, controlled in 25 (38%) of 66, and resolved in 25 (38%) of 66 patients. Among patients with active AICs at the start of treatment with ibrutinib, the treatment induced an improvement of the AICs in 6 patients (2 received concomitant steroid treatment and 1 received steroids along with rituximab) and a resolution in 9 patients (2 received concomitant steroids), whereas in 1 patient, the ITP remained stable. Controlled AICs resolved in 12 of 25 patients, improved in 8, and remained stable in 5 (in 1 patient, steroids were also administered). Within these 2 subgroups of ibrutinib-treated patients, 4 patients were already receiving steroids when treatment with ibrutinib was started, and 3 of them were able to taper off the steroids. None of the 25 patients with a resolved AIC when treatment with ibrutinib was started experienced an AIC flare during treatment.
Evolution of preexisting AICs during treatment with a targeted drug. The status of preexisting AICs at the start of treatment with a targeted drug and their evolution during therapy with ibrutinib (A), idelalisib (B), and venetoclax (C) is depicted. In each group of patients, categorized on the basis of AIC status (ie, resolved, controlled, or active) when the targeted drug was started, the numbers of patients showing different outcomes (ie, resolved, improved, stable, and worsening) are indicated. The tables below each panel report, for each subgroup indicated by symbols, the number of patients receiving additional AIC-directed therapies and the type of concomitant immunosuppressive regimen.
Evolution of preexisting AICs during treatment with a targeted drug. The status of preexisting AICs at the start of treatment with a targeted drug and their evolution during therapy with ibrutinib (A), idelalisib (B), and venetoclax (C) is depicted. In each group of patients, categorized on the basis of AIC status (ie, resolved, controlled, or active) when the targeted drug was started, the numbers of patients showing different outcomes (ie, resolved, improved, stable, and worsening) are indicated. The tables below each panel report, for each subgroup indicated by symbols, the number of patients receiving additional AIC-directed therapies and the type of concomitant immunosuppressive regimen.
When treatment with idelalisib was initiated, the preexisting AIC was considered active in 9 (31%) of 29, controlled in 11 (38%) of 29, and resolved in 9 (31%) of 29 patients. During treatment with idelalisib, AICs resolved in 3 patients with active AICs and in 7 patients with controlled AICs (steroids were also given in 1 patient). Preexisting AICs improved in 3 patients with active AICs (in 1 patient, steroids, intravenous IG [IVIG], and thrombopoietin mimetics were also given) and in 2 patients with controlled AICs. Two additional patients with active AICs when treatment with idelalisib was started remained stable, and 1 patient with a controlled AIC initially responded but had a later AIHA flare in concomitance with CLL relapse (after 25 months of therapy). One patient with controlled AICs and 1 with active preexisting AICs had a worsening of the AIHA, in spite of the addition of steroids. Overall, 2 patients were already receiving steroids when treatment with idelalisib was started, and 1 of them was able to taper off the steroids. No AIC recurrence was observed in patients who had a resolved AIC when treatment with idelalisib was started.
In the venetoclax cohort, when treatment was started, the preexisting AIC was active in 2 (22%) of 9, controlled in 1 (11%) of 9, and resolved in 6 (67%) of 9 patients. Active AICs resolved with venetoclax treatment in 1 patient and improved, albeit with concomitant steroid therapy, in the second patient. The patient with a controlled AIC remained stable but needed additional AIC-directed therapy (ie, steroids and rituximab). Of the 2 patients who were already receiving steroids when venetoclax was started, 1 was able to taper off the steroids. Among the 6 patients with resolved AICs, 1 had an AIHA recurrence after 4 months from the start of treatment with venetoclax, which was successfully managed by interrupting venetoclax and administering steroids.
Treatment-emergent AICs
Treatment-emergent AICs occurred in 5 (1%) of 506 patients during ibrutinib therapy, in 1 (0.9%) of 114 patients during idelalisib therapy, and in 6 (7%) of 91 patients during venetoclax therapy (Table 4). Of note, the evaluation of treatment-emergent AICs was carried out by analyzing only patients who did not present any episode of AICs before targeted treatment was started. The estimated incidence rate of treatment-emergent AICs during targeted drug therapy was 5 episodes per 1000 patients per year of ibrutinib exposure (95% confidence interval [CI], 2-10 episodes), 6 episodes per 1000 patients per year of idelalisib exposure (95% CI, 0-29 episodes), and 69 episodes per 1000 patients per year of venetoclax exposure (95% CI, 28-143 episodes). In this analysis, a comparison among treatment groups showed that the incidence rate of treatment-emergent AICs during venetoclax treatment was significantly higher compared with treatment with ibrutinib or idelalisib (P ≤ .001 for both comparisons). The higher incidence of treatment-emergent AICs in the venetoclax cohort compared with the ibrutinib and idelalisib groups was maintained when the analysis was restricted to only those patients with relapsed or refractory CLL or to patients who had previously received ≥2 lines of therapy (supplemental Tables 5 and 6) and also when only the first year of therapy for all patients included in the 3 treatment cohorts was considered (supplemental Table 7).
The median time of AIC occurrence from the start of treatment with the targeted drug was 3 months for ibrutinib (range, 0-29 months) and 5 months for venetoclax (range, 3-15 months). In the idelalisib cohort, the only treatment-emergent AIC episode (patient 6) occurred after 25 months of therapy. In 3 patients, 1 in each treatment group (patients 5, 6, and 12), treatment-emergent AICs developed concomitantly with or shortly before CLL progression was detected.
In our enitre cohort, patients who developed treatment-emergent AICs were predominantly males (11 [92%] of 12), had a median age of 70 years (range, 50-82 years), and had unfavorable biological features such as unmutated IGHV (9 of 9 patients for whom the data were available) and del(17p) and/or TP53 mutation (10 [83%] of 12). A minority of patients who developed treatment-emergent AIHA had baseline direct antiglobulin test positivity (patients 1, 3, and 7), whereas in 6 (67%) of 9 patients, baseline direct antiglobulin test was negative (patients 2, 5, 6, 8, 11, and 12). Interestingly, none of the patients who developed treatment-emergent AICs during ibrutinib or venetoclax therapy were receiving the targeted drug in association with an anti-CD20 monoclonal antibody.
When a treatment-emergent AIC occurred, the targeted drug was continued in 5 of 12 patients, whereas it was temporarily held or dose-reduced in 5 of 12 patients; in 2 of 12 patients the targeted drug was definitively discontinued because of AICs (1 of them, patient 5, had concomitant CLL progression). AICs were managed with the addition of steroids alone in 6 patients with AIHA and in 1 patient with ITP, rituximab alone in 1 patient with AIHA, steroids and rituximab in 1 patient with AIHA, and steroids and IVIG with or without rituximab in 2 patients with ITP. Overall, despite AICs, 10 of 12 patients were able to continue the treatment with the targeted drug (in 1 patient at a stable reduced dose), but 2 of them discontinued therapy shortly after the treatment-emergent AIC because of disease progression (patients 6 and 12). Among the patients who continued the targeted drug, the AIC event was considered resolved in 6 patients and controlled in 4.
Discussion
This study provides the largest analysis to date regarding the impact of treatment with ibrutinib, idelalisib, or venetoclax on the natural history of CLL-associated AICs. We observed that the majority of AICs that were not resolved when the targeted drug treatment was started had an improvement or a resolution during therapy, whereas only in a few patients the AICs worsened or remained stable. We also showed that the occurrence of treatment-emergent AIC episodes during the administration of targeted drugs in patients without a known history of preexisting AICs is a rare event, which in most patients is manageable without requiring the interruption of treatment.
The centers participating in this study were academic institutions, tertiary care hospitals, and regional hospitals, in which all patients were followed by physicians highly experienced in managing CLL, thus allowing efficacy data previously reported in the literature to be reproduced. In addition, although with the limitations of a retrospective analysis, the relevance of our findings was strengthened by the application of strict predefined criteria to define AIC status and outcome.
Overall, the 13% rate of preexisting AICs observed in our cohort is in line with data previously reported for patients treated with ibrutinib.19,20 A consistently higher frequency (26%) was reported only by Rogers et al,21 but in that cohort, patients were at an increased risk for AICs, given the higher median number of previous therapies.
Data from the literature tend to associate AICs with CLL unfavorable prognostic parameters and to occasionally attribute a negative impact on OS to autoimmune manifestations.32 In our study, the presence of preexisting AICs was significantly associated with adverse FISH results and with a history of >2 previous lines of therapy. However, when treatment groups were analyzed separately, adverse FISH results remained significant only in the venetoclax group, and pretreatment status remained significant only in the ibrutinib group. A preexisting AIC did not have a significant impact on PFS and OS. This observation differs from some of the previous reports at least partly because of the characteristics of our cohort; it was markedly enriched for high-risk patients, which may have attenuated the strength of the association of preexisting AICs with negative prognostic factors and with adverse prognosis. Interestingly, in both the entire cohort and the largest ibrutinib-treated group, we observed an association of preexisting AICs with severe hypogammaglobulinemia, which could be the hallmark of a more aggressive or advanced disease, but could also be the expression of a more dysregulated immune system.
Among patients with preexisting AICs that had already resolved when treatment with the targeted drug was started, we observed only 1 recurrence, which occurred at 4 months after venetoclax treatment was initiated, and improved after drug treatment was interrupted and steroids were administered. During treatment with targeted drugs, there was an overall tendency toward AIC recovery in patients with controlled or active autoimmune phenomena, and only a few patients needed additional AIC-directed therapy. This beneficial effect, which is consistent with previously reported experiences in patients treated with ibrutinib and idelalisib,20-22 seems to be associated with effective control of the underlying CLL. However, additional data should be obtained to better discriminate between an off-tumor effect of each targeted drug on the immune system and an immune recovery consequent to the tumor burden reduction.
When we compared the 3 treatment cohorts, the percentage of patients whose AICs improved during therapy (AICs categorized as resolved or improved among those that were active or controlled at the start of treatment) was higher in the ibrutinib group (85%) compared with the idelalisib (75%) and venetoclax (66%) groups. However, it is difficult to draw a definitive conclusion on a more beneficial effect of ibrutinib in controlling CLL-associated AICs, mainly because of the smaller number and shorter follow-up of patients in the venetoclax cohort, and to the less durable efficacy of idelalisib in controlling the underlying CLL.
Historically, different CLL-directed drugs, in particular fludarabine used as a single agent, have been reported as being capable of triggering AICs.33-35 In our analysis, we observed a very low prevalence of treatment-emergent AICs (1%) during treatment with ibrutinib. Our data are in line with previous findings from clinical trials, as shown by the absence of new cases of AICs in the RESONATE study,19 and by the occurrence of only 2% of treatment-emergent AICs in the study by Rogers et al.21 In the real-life setting, Hampel et al20 reported a higher rate (6%), but in their study, relapsed patients were defined as having treatment-emergent AICs. With a cohort of 572 patients, our study represents the largest reported retrospective analysis specifically addressing the impact of ibrutinib on AICs.
Among patients treated with idelalisib, we found that 0.9% developed treatment-emergent AICs. In the absence of previously published studies reporting on the occurrence of AICs during treatment with idelalisib-based regimens, our report adds a relevant piece of information and suggests that treatment-emergent AICs might be marginal in this setting. This notion is also supported by data from the phase 3 pivotal study of idelalisib and rituximab in relapsed or refractory CLL, in which AIC is not mentioned among the most frequent AEs.23,36
Notably, the rate of treatment-emergent AICs in our study was higher in the venetoclax cohort compared with the ibrutinib and idelalisib cohorts. Similar rates of treatment-emergent AICs had previously been reported in patients treated with venetoclax within clinical trials. In the phase 1/2 trial for relapsed or refractory CLL, 3% of patients presented with a serious ITP,28 and in the phase 2 trial enrolling CLL patients with del(17p), AIHA occurred in 8% (grade ≥3, 7%) and ITP occurred in 5% (all grade ≥3) of patients.37
With the aim of correcting for a possible bias induced by the different pretreatment characteristics of patients enrolled in the 3 cohorts, we also assessed the treatment-emergent AIC incidence rate in patients grouped on the basis of their CLL pretreatment status. We confirmed the higher incidence of treatment-emergent AICs in patients undergoing venetoclax treatment compared with the ibrutinib and idelalisib groups when only patients with relapsed or refractory disease or patients who had previously received ≥2 lines of therapy were considered in each cohort. Although a direct comparison between treatment groups is prevented by the heterogeneity of the patients included in the 3 cohorts and by the small number of patients with active AICs, these data, together with the above-mentioned observations from clinical trials, draw attention to a possible impact of venetoclax in eliciting AICs episodes.
The observation that treatment-emergent AICs did not develop in those patients who were receiving ibrutinib or venetoclax in combination with rituximab or obinutuzumab, together with the low proportion of treatment-emergent AICs occurring in patients receiving idelalisib (always administered in combination with rituximab) suggest that the addition of an anti-CD20 monoclonal antibody might add an immunosuppressive protective effect, preventing the development of AIC episodes. In the phase 3 Murano trial, only 3 patients discontinued treatment because of AICs in the venetoclax and rituximab arm.30 In patients treated with venetoclax and obinutuzumab in the CLL14 trial, AICs were not reported among common AEs.38 This hypothesis certainly warrants further evaluation in larger prospective series, and from now on, the wider use of venetoclax in combination with anti-CD20 monoclonal antibodies in clinical practice will certainly help assuage possible concerns regarding venetoclax-induced AICs.
The restricted number of treatment-emergent AICs observed in our study limits our ability to draw definitive conclusions on the association between treatment-emergent AICs and other CLL characteristics. However, we can highlight that all patients in our cohort had unmutated IGHV, and the majority had del(17p) and/or a TP53 mutation. This is in line with previous data from Hampel et al20 and again seems to support the notion of an association between autoimmune phenomena and averse disease characteristics in CLL.
In our patients, the time of treatment-emergent AIC occurrence was variable. Notably, late-onset autoimmune events were commonly associated with CLL disease already progressing or anticipated to progress soon, and no events occurred in patients who achieved a CR. AIC management was heterogeneous, which reflects the lack of specific guidelines to help physicians choose a treatment approach in these specific situations. However, the overall outcome of treatment-emergent AICs proved favorable, especially when patients with autoimmune episodes associated with a loss of response to the targeted drug are not considered.
In summary, ibrutinib, idelalisib, and venetoclax have a beneficial impact on CLL-related preexisting AICs, achieving in most patients, in parallel with the consolidated antitumor efficacy, an effective control of the autoimmune phenomena. Overall, the incidence of treatment-emergent AICs is negligible in patients treated with ibrutinib or idelalisib, whereas it seems more meaningful in patients treated with venetoclax. However, the risk of AIC episodes should not limit the use of venetoclax, considering the strong efficacy of this drug in treating patients with CLL, including those with high-risk features, and the possibility of effectively managing autoimmune complications, mostly without treatment interruption.
For original data, please send an e-mail to Marta Coscia at marta.coscia@unito.it.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Fondazione European Myeloma Network (EMN) Italy Onlus, and L.T. and A.V. thank “Ricerca per Credere nella Vita” Organismo di Vigilanza (ODV; Padua, Italy).
This work was partly supported by the University of Torino (local funds ex-60%) (M.C.), by Fondo di Ateneo per la Ricerca (F.A.R. 2018 and 2019, University of Ferrara) (G.M.R. and A.C.), by the BEAT Leukemia Foundation (A.C.), by the Associazione Italiana Ricerca sul Cancro (AIRC) and by Special 5x1000 Program Metastases (21198; Milan, Italy) (G.G. and R.F.).
C.V. was the recipient of a fellowship from Associazione Italiana contro le Leucemie, Linfomi e Mieloma (A.I.L.) and a “Fondazione Pezcoller-Ferruccio ed Elena Bernardi” fellowship from Pezcoller Foundation, in collaboration with Società Italiana di Cancerologia (S.I.C.). V.G. was the recipient of a fellowship from the Fondazione Cassa di Risparmio di Torino, a fellowship from AIRC (“Anna Nappa” fellowship, ref. no. 16343),and a “Giorgio Bissolotti e Teresina Bosio” fellowship from Fondazione “Angela Bossolasco."
Authorship
Contribution: C.V. and M.C. designed the research, interpreted the data, and wrote the manuscript; C.V. and C.S. provided patient care and collected and analyzed data; M.C. provided patient care and supervised the study; V.G., M.P., L. Schiattone, G.Z., A.V., F.V., R.C., G.M.R., R.M., L.L., P.R., M.M., E.P., M.G., E.B., F.P., M.C.M., L.D.P., G.R., L.O., L.T., A.C., A.T., and L. Scarfò collected data and provided patient care; G.G., F.R.M., R.F., and M.B. contributed to data interpretation; and all authors discussed results, contributed to manuscript revision, and approved the final manuscript.
Conflict-of-interest disclosure: C.V. received consultancy fees from Janssen outside the submitted work. A.V. received fees from Janssen, AbbVie and Gilead outside the submitted work. G.M.R. received honoraria from AbbVie, Gilead, and Janssen and research funding from Gilead outside the submitted work. L.L. received honoraria and research funding from AbbVie and Roche and honoraria from Gilead and Janssen outside the submitted work. G.R. received honoraria for speaker’s conferences and advisory boards for Janssen, AbbVie, Gilead, and Takeda outside the submitted work. L.T. received research funding from Janssen and Gilead and fees from Janssen, AbbVie, AstraZeneca, Roche, and Takeda outside the submitted work. A.C. received honoraria from AbbVie, AstraZeneca, Gilead, and Janssen outside the submitted work. L. Scarfò received honoraria from AbbVie, AstraZeneca, Gilead, and Janssen outside the submitted work. R.F. received honoraria for advisory boards and/or a speaker’s bureau from Janssen, AbbVie, Amgen, Novartis, Shire, and Incyte outside the submitted work. M.B. received personal fees from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb, and AbbVie and research funding from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol Myers Squibb, and Mundipharma outside the submitted work. M.C. received research funding from Janssen and Karyopharm Therapeutics and personal fees from Janssen, Gilead, AbbVie, and Shire outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Marta Coscia, Division of Hematology, Azienda Ospedaliero–Universitaria Città della Salute e della Scienza di Torino, Via Genova 3, 10126 Torino, Italy; e-mail: marta.coscia@unito.it.