Abstract
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is a rare lymphoma entity with distinct pathologic and clinical characteristics. Unlike the malignant cells in classical Hodgkin lymphoma, the disease-defining lymphocyte-predominant cells in NLPHL are consistently positive for CD20, but do not express CD30. The clinical course of NLPHL is indolent in the majority of cases. Most patients present with early-stage disease at the initial diagnosis. First-line treatment of stage IA NLPHL usually consists of limited-field radiotherapy alone. Patients with early-stage NLPHL other than stage IA and intermediate-stage disease mostly receive combined-modality treatment, whereas individuals with advanced NLPHL are treated with chemotherapy alone. In relapsed NLPHL, conventional chemotherapy, anti-CD20 antibodies, and radiotherapy represent active treatment modalities. Only patients with poor-risk characteristics such as early disease recurrence are candidates for aggressive salvage treatment with high-dose chemotherapy and autologous stem cell transplantation. The overall and relative survival of patients with NLPHL is excellent as indicated by a low excess mortality compared with the general population. This article discusses treatment options for patients with NLPHL and factors that influence the choice of therapy on the basis of the available data and 2 clinical cases.
Introduction
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is a rare lymphoma entity accounting for ∼5% of Hodgkin lymphoma cases.1 The disease most often affects middle-aged men.2,3 Pathologic and clinical characteristics of NLPHL differ significantly from those of classical Hodgkin lymphoma (cHL). In contrast to the malignant Hodgkin and Reed-Sternberg cells in cHL, the disease-defining lymphocyte predominant cells in NLPHL are consistently positive for CD20, but do not express CD30. Histopathologically, NLPHL can be divided into cases with a typical growth pattern (∼75% of cases) and cases with a variant histology (∼25% of cases).4-6 The group allocation depends on the localization of the lymphocyte predominant cells within the affected tissue and the composition of the microenvironment. Cases presenting with a variant histology are sometimes difficult to distinguish from aggressive B-cell non-Hodgkin lymphoma (B-NHL).4 The histopathologic growth pattern has some prognostic impact because variant histology is associated with more advanced disease, a higher overall relapse rate, and an increased rate of early relapses.6,7 Nonetheless, the histopathologic growth pattern has not influenced the choice of NLPHL treatment thus far.
At the initial diagnosis, the majority of patients with NLPHL have early-stage disease. The clinical course is usually indolent despite a tendency toward late relapses and histologic transformation into aggressive B-NHL.8-10 The prognosis of patients with NLPHL is excellent as indicated by a low excess mortality compared with the general population.11 Among patients with a history of NLPHL, lymphoma does not represent the major cause of death.8,12
The present article discusses treatment options for patients with NLPHL on the basis of the available data and reviews factors that may help in choosing the optimal therapy for the individual patient.
Case 1
A 31-year-old man with no relevant comorbidities presented with an enlarged lymph node in the left axilla. Initially, he had noticed the lymph node almost 1 year before the consultation. No clinical symptoms were reported. The lymph node was resected, and NLPHL was diagnosed. Staging procedures, including computed tomography (CT) scan of the neck, chest, and abdomen as well as bone marrow aspiration and biopsy, were performed. Except for the left axilla, no sites were affected, and thus the patient had stage IA disease. He was treated with 4 weekly doses of rituximab within a prospective study.13 A complete remission (CR) was achieved. However, after 5 years, the patient presented again with an enlarged lymph node in the left axilla. The patient underwent lymph node resection, and the diagnosis of NLPHL recurrence with a typical histopathologic growth pattern was made. Staging procedures revealed stage IIA disease involving the left cervical and axillary lymph nodes. The treatment options discussed with the patient included stage-adapted combined-modality treatment (CMT) with 2 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by limited-field radiotherapy (RT) and single-agent anti-CD20 antibody therapy with ofatumumab within a phase 2 study.14 The patient consented to participate in the study and received 8 weekly doses of the antibody, resulting in a second CR that has been ongoing for more than 6 years.
Case 2
A 27-year-old man presented with an enlarged inguinal lymph node that had developed over the previous 2 weeks. He also had lost weight and had fever and night sweats. The inguinal lymph node was resected, and NLPHL was diagnosed. According to the staging, including CT scan of the neck, chest, and abdomen as well as bone marrow aspiration and biopsy, the patient had stage IIB disease with no additional clinical risk factors. He was included in a prospective study for patients with early-stage Hodgkin lymphoma and received 2 cycles of ABVD followed by involved-field RT.15 Treatment resulted in a CR. However, 18 months later, the patient presented with fever, night sweats, and generalized lymphadenopathy. Diagnostic and staging procedures, including the biopsy of an iliac lymph node, revealed stage IVB NLPHL (generalized lymphadenopathy, involvement of liver, bone marrow, and spleen) with a variant histology. Salvage therapy consisted of 2 cycles of rituximab, dexamethasone, high-dose cytarabine, and cisplatin (R-DHAP) followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT). The patient responded to treatment and was still in remission at his follow-up visit 2 years after the end of second-line treatment.
Treatment of stage IA NLPHL with no clinical risk factors
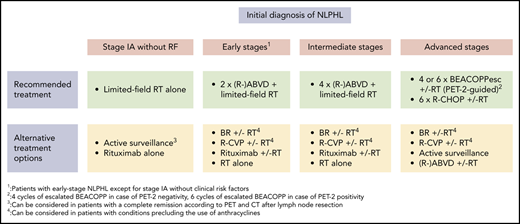
At most institutions, limited-field RT alone represents the standard of care for patients with stage IA NLPHL with no clinical risk factors (Table 1; Figure 1). Several retrospective analyses have consistently shown that this approach is equally effective and less toxic compared with CMT. The largest study addressing this issue came from the German Hodgkin Study Group (GHSG). Patients had received CMT (n = 72), extended-field RT (n = 49), or involved-field RT (n = 108). At 8 years, progression-free survival (PFS) and overall survival (OS) rates were comparable for all treatment groups (CMT, 88.5% and 98.6%, respectively; extended-field RT, 84.3% and 95.7%; and involved-field RT, 91.9% and 99.0%).12 Patients who had CMT more often developed grade III/IV acute toxicities.16 Another analysis conducted in the United States included 93 patients with limited-stage NLPHL who had received RT alone.17 The 10-year PFS and OS rates for stage I patients were 89% and 96%. In agreement with the GHSG study, PFS and OS did not differ between patients who had involved-field RT and patients who had RT to more extended fields. The question of whether a further reduction of the RT field is possible without a loss of disease control was subject to a recent analysis comprising 36 patients with limited-stage NLPHL who had extended-field RT (n = 9), involved-field RT (n = 13), or involved-site RT (n = 14).18 Disease control was excellent irrespective of the RT field. Thus, involved-site RT alone is likely sufficient for patients with stage IA NLPHL with no clinical risk factors; final conclusions are difficult to make, however, due to the low number of patients included in the analysis and the limited median follow-up of only 2.6 years for the involved-site RT group.
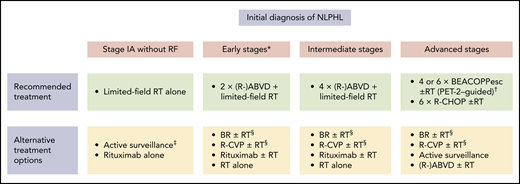
Treatment options in newly diagnosed NLPHL. *Patients with early-stage NLPHL except for stage IA with no clinical risk factors. †Four cycles of escalated BEACOPP (BEACOPPesc) in case of interim PET after 2 cycles of chemotherapy with escalated BEACOPP (PET-2) negativity, 6 cycles of BEACOPPesc in case of PET-2 positivity. ‡Can be considered in patients with a CR according to PET and CT imaging after lymph node resection. §Can be considered in patients with conditions precluding the use of anthracyclines.
Treatment options in newly diagnosed NLPHL. *Patients with early-stage NLPHL except for stage IA with no clinical risk factors. †Four cycles of escalated BEACOPP (BEACOPPesc) in case of interim PET after 2 cycles of chemotherapy with escalated BEACOPP (PET-2) negativity, 6 cycles of BEACOPPesc in case of PET-2 positivity. ‡Can be considered in patients with a CR according to PET and CT imaging after lymph node resection. §Can be considered in patients with conditions precluding the use of anthracyclines.
Although the rate of significant toxicities is low among patients receiving limited-field RT alone, different studies aimed to minimize the treatment burden for individuals with stage IA NLPHL with no clinical risk factors. A GHSG phase 2 study including 28 patients investigated the first-generation anti-CD20 antibody rituximab.13 Four weekly standard doses were given. All patients responded to treatment, but long-term disease control was poor, with a 10-year PFS rate of 51.1%. However, because relapses could be salvaged successfully, the 10-year OS rate was 91.0% and thus comparable to RT alone.19 Active surveillance was prospectively evaluated in 52 children with stage IA NLPHL restricted to a single lymph node who had achieved a CR after lymph node resection as confirmed by positron emission tomography (PET) and CT imaging. The 5-year event-free survival and OS estimates were 77.1% and 100%, respectively.20 Despite these encouraging data, it must be remembered that this approach is only applicable in a highly selected subgroup of patients. In addition, there might be slight differences between children and adult patients.
Taken together, the long-term survival rate of patients with stage IA NLPHL with no clinical risk factors is in excess of 90% irrespective of the applied treatment. However, we consider limited-field RT alone at a dose of 30 Gy as the treatment of choice in this situation because it not only results in an excellent long-term disease control but is associated with a low rate of toxicities. For patients who are not eligible for or refuse RT, active surveillance in case of a CR after lymph node surgery or single-agent anti-CD20 antibody treatment can be discussed as alternative approaches (Figure 1).
Treatment of NLPHL in early stages other than stage IA and intermediate stages
Patients with early-stage NLPHL other than stage IA with no clinical risk factors and intermediate-stage NLPHL usually receive CMT (Table 1; Figure 1). Compared with RT alone, CMT results in a better disease control in these patients. A retrospective study using the British Columbia Cancer Agency (BCCA) database included 88 patients with early-stage NLPHL who had received RT alone (n = 32) or CMT (n = 56). Chemotherapy in the CMT group consisted of 2 cycles of ABVD in the majority of cases. At 10 years, PFS rates were 91% for the CMT group and 65% for the RT-alone group. Thus, the addition of chemotherapy seemed to result in a significant improvement in disease control. The 10-year OS rates after CMT and RT alone were similar.21 A recent analysis from the GHSG included 251 patients with early-stage NLPHL and 76 patients with intermediate-stage disease.8 Treatment consisted of 2 or 4 cycles of ABVD or ABVD-based protocols followed by consolidation RT in most cases. The 10-year PFS and OS estimates were 75.5% and 92.1% for patients with early-stage disease and 72.1% and 96.2% for patients with intermediate-stage NLPHL. Whether the addition of an anti-CD20 antibody to ABVD chemotherapy results in better disease control remains unclear as data on the combination treatment are limited and derive from small case series with short follow-up.22,23
In summary, the available data suggest that a brief chemotherapy with 2 cycles (early stages other than stage IA with no clinical risk factors) or 4 cycles (intermediate stages) of ABVD followed by limited-field RT at 20 Gy (early stages other than stage IA without clinical risk factors) or 30 Gy (intermediate stages) should be the preferred first-line approach for NLPHL patients with early-stage disease other than stage IA with no clinical risk factors and intermediate-stage disease. In patients who are not eligible for chemotherapy with ABVD due to heart disease, anthracycline-free protocols such as bendamustine and rituximab (BR) and rituximab, cyclophosphamide, vinblastine, and prednisone (R-CVP), as well as single-agent anti-CD20 antibody treatment and RT alone, can be considered; the data are scarce, however (Figure 1).24-27
Treatment of NLPHL in advanced stages
Newly diagnosed advanced NLPHL is usually treated with conventional chemotherapy optionally combined with an anti-CD20 antibody (Table 1; Figure 1). Consolidation RT is restricted to individuals with larger residual lymphoma at the end of systemic treatment. A retrospective GHSG analysis included 144 patients with advanced NLPHL who had mostly received escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), BEACOPP baseline, or BEACOPP-14. The 10-year PFS and OS estimates were 69.8% and 87.4%, respectively.8 A more recent analysis included 86 patients with advanced NLPHL who had treatment within the randomized GHSG HD18 study that evaluated treatment stratification on the basis of interim PET imaging after 2 cycles of escalated BEACOPP.28 After a median observation time of 63 months, the overall 5-year PFS rate was 82.8%. Patients with a positive PET scan after 2 cycles of chemotherapy had a 5-year PFS rate of 70.2%, whereas the 5-year PFS rate for patients with a negative PET scan after 2 cycles of escalated BEACOPP was 90.4%. The reduction of treatment to a total of only 4 cycles of escalated BEACOPP did not seem to result in a loss of disease control in patients with a negative interim PET scan. Thus, the GHSG standard approach for patients with advanced cHL consisting of a total of 6 cycles of escalated BEACOPP for patients with a positive interim PET scan and a total of 4 cycles of escalated BEACOPP for patients with a negative PET scan after 2 cycles of chemotherapy also results in very good outcomes among patients with advanced NLPHL. Despite the possibility of reducing treatment to a total of only 4 cycles of chemotherapy in a significant proportion of patients, it is unclear whether all individuals with advanced NLPHL benefit from intensive BEACOPP-based therapy given the significant acute toxicity and the possibly increased risk for the development of severe late effects associated with this protocol.29-32
ABVD and ABVD-like regimens are associated with a higher rate of lymphoma recurrence with either NLPHL histology or histologic transformation into aggressive B-NHL than BEACOPP-based protocols. A retrospective analysis using the BCCA database included 42 patients with newly diagnosed advanced NLPHL who had chemotherapy with ABVD or ABVD-like protocols.33 At 10 years, the lymphoma recurrence rate was ∼40%. However, OS was still excellent with a 10-year rate of 83.5%.
Smaller studies retrospectively evaluated the rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and BR protocols in NLPHL. An analysis conducted at a single center in the United States included 14 patients with advanced NLPHL who had been treated with R-CHOP.34 The PFS estimates at 5 and 10 years were each 85.7%. A case series included 9 patients with NLPHL who had received BR.25 Among those, 8 had advanced-stage disease. All patients responded to treatment, and after a median observation time of 34 months, no case of disease recurrence had occurred. Although these data are promising, final conclusions regarding the role of R-CHOP and BR in the treatment of NLPHL cannot yet be drawn due to the low number of patients included in the available analyses.
In an attempt to reduce treatment toxicity, single-agent rituximab was investigated in a phase 2 study comprising patients with newly diagnosed and relapsed NLPHL.35 Patients received 4 weekly standard doses of the antibody either alone or followed by rituximab maintenance every 6 months for 2 years. All patients responded to treatment. However, the 5-year PFS rates for patients with newly diagnosed NLPHL (n = 21) receiving rituximab induction alone and rituximab induction followed by rituximab maintenance were 41.7% and 51.9%, respectively. Despite the increased relapse rate, OS was not impaired.
Generally, the choice of treatment of patients with advanced NLPHL can be difficult, and a number of factors, including disease burden, affected sites, clinical course, and histopathological growth pattern, should be taken into consideration for the treatment decision. Based on the available evidence, we usually apply interim PET-guided treatment with escalated BEACOPP as the first-line approach in advanced NLPHL. This approach allows a treatment reduction to only 4 cycles of chemotherapy for most patients.36 However, given the significant acute toxicity and the potential risk for the development of therapy-related late effects such as second malignancies and infertility, a relevant proportion of patients may not be candidates for escalated BEACOPP.31,32 The preferred alternative protocol at our institution is R-CHOP. Other regimens, including a relevant dose of alkylating agents such as BR or R-CVP, can also be considered because alkylating agents seem to play an important role for disease control in NLPHL.37 In patients with advanced NLPHL presenting with a low lymphoma burden and an indolent clinical course, active surveillance might also be discussed as the initial management strategy (Figure 1).38
Treatment of relapsed NLPHL
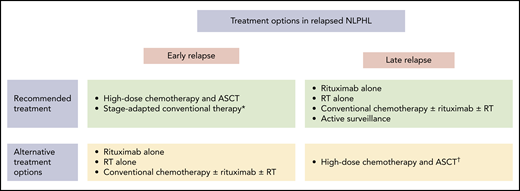
Treatment of relapsed NLPHL should be determined individually based on various factors (Figure 2). Most patients with NLPHL recurrence are candidates for nonaggressive salvage approaches such as single-agent anti-CD20 antibody treatment, RT alone, and conventional chemotherapy optionally combined with an anti-CD20 antibody and RT. These treatment modalities should be considered, particularly in patients who had early-stage NLPHL at initial diagnosis and thus first-line treatment with RT alone or limited amounts of chemotherapy, patients with a low disease burden at relapse, individuals with an indolent clinical course, and those with a long duration of response after first-line treatment. According to a retrospective analysis from the GHSG, including 99 patients with NLPHL recurrence, most patients had received one of these nonaggressive approaches at relapse. The 5-year PFS and OS rates estimated from the date of disease recurrence were 74.1% and 97.2% for patients treated with single-agent anti-CD20 antibody treatment or RT alone and 68.0% and 77.8% for patients who had conventional chemotherapy optionally combined with an anti-CD20 antibody and RT.9 Other studies also revealed good outcomes after salvage treatment with an anti-CD20 antibody. A phase 2 study including 15 patients with NLPHL recurrence reported a response rate of 94% after 4 weekly standard doses of rituximab.39 After a median observation time of 63 months, the median time to progression was 33 months. Only 1 patient died during follow-up. The second-generation anti-CD20 antibody ofatumumab was investigated in a phase 2 study comprising 28 patients with relapsed NLPHL.14 Study treatment consisted of 8 weekly doses of the antibody. The response rate was 96%. After a median follow-up of 26 months, the 2-year PFS and OS estimates were 80% and 100%. In case conventional chemotherapy is applied at NLPHL recurrence, all protocols that are active in newly diagnosed disease can generally be applied. Those include ABVD, BEACOPP, R-CHOP, BR, and R-CVP.8,21,24,25,34 The choice of the optimal protocol for the individual patient should allow for prior therapies, disease burden at relapse, and patient characteristics such as age and comorbidities.
Treatment options in relapsed NLPHL. *Can be considered in patients who had RT alone, rituximab alone, active surveillance, or CMT containing limited amounts of chemotherapy as management strategy at the initial NLPHL diagnosis. †Should be considered especially in patients with poor-risk characteristics.
Treatment options in relapsed NLPHL. *Can be considered in patients who had RT alone, rituximab alone, active surveillance, or CMT containing limited amounts of chemotherapy as management strategy at the initial NLPHL diagnosis. †Should be considered especially in patients with poor-risk characteristics.
The use of high-dose chemotherapy and ASCT should be restricted to poor-risk patients with NLPHL recurrence after chemotherapy-based first-line treatment. This group of patients comprises individuals with early relapse less than 2 years after the initial NLPHL diagnosis and those with liver or bone marrow involvement because these factors were shown to be associated with an impaired OS.8 In addition, high-dose chemotherapy and ASCT should be considered in patients with an aggressive clinical course. The largest analysis on high-dose chemotherapy and ASCT came from the European Society for Blood and Marrow Transplantation.40 A total of 60 patients with relapsed NLPHL were included. The median time interval between the initial NLPHL diagnosis and ASCT was 21 months. Most patients had advanced disease at relapse. After a median follow-up of 56 months, the 5-year PFS and OS estimates were 66% and 87%, respectively, and thus excellent. Similar results were obtained from smaller analyses also investigating high-dose chemotherapy and ASCT in patients with NLPHL recurrence.9,41
At our institution, conventional salvage approaches are applied in the vast majority of patients with relapsed NLPHL. Only patients with poor-risk characteristics are treated with high-dose chemotherapy and ASCT.
Treatment of histologic transformation into aggressive B-NHL
The incidence, treatment, and course of histological transformation into aggressive B-NHL have been evaluated in several retrospective analyses. A registry-based study from France included 164 patients with NLPHL who mostly had first-line treatment with ABVD. Histologic transformation occurred in 19 patients. The median time to histologic transformation was 4.7 years, and the 10-year cumulative transformation rate was 12%. Treatment at histologic transformation consisted of conventional chemotherapy in 10 patients and high-dose chemotherapy and ASCT in 9 patients. The 10-year OS rate from the time of histologic transformation was 60%.424 Similar data came from an analysis using the BCCA database. A total of 95 patients with the initial diagnosis of NLPHL were taken into account. The actuarial risk for histological transformation was 7% at 10 years. Individuals with splenic involvement at NLPHL diagnosis were at an increased risk for the development of histologic transformation. At histologic transformation, most patients received a salvage protocol including an anti-CD20 antibody followed by consolidation high-dose chemotherapy and ASCT. The 10-year PFS and OS rates were 52% and 62%, respectively.10 A recent multi-institutional study from the International Lymphoma Radiation Oncology Group comprising 559 patients initially diagnosed with stage I/II NLPHL reported a transformation rate of 3.8% after a median follow-up of 5.5 years. In these patients who only had limited amounts of treatment of NLPHL, R-CHOP was most frequently given at histologic transformation. The 5-year PFS and OS estimates from the diagnosis of aggressive B-NHL were 62.2% and 88.4%.27
In line with these data, we usually apply R-CHOP or high-dose chemotherapy and ASCT to treat patients who develop histologic transformation into aggressive B-NHL.
Follow-up of case 1
This case displays several points that are typical for NLPHL. Patients with NLPHL mostly present with limited-stage disease at initial diagnosis. Single-agent anti-CD20 antibody treatment usually induces a remission, but disease recurrence occurring late after first-line treatment is common.13,14 However, salvage therapy with nonaggressive approaches is successful in most cases. Thus, individuals with NLPHL generally have a good prognosis without necessitating intensive treatment even in case of relapse.9 This outcome is particularly true for patients with a typical histopathologic growth pattern who mostly present with an indolent clinical course.
Follow-up of case 2
This case illustrates an atypical course of NLPHL. Initial presentation with B symptoms and disease recurrence less than 2 years after the initial diagnosis is seen in few patients. These patients often present with a variant histology and have a poor outcome. This is especially true for cases with liver or bone marrow involvement.8 Aggressive second-line treatment was therefore chosen in this patient.
Conclusions
Treating NLPHL represents a challenge because the data are limited due to the low incidence of the disease. Most knowledge regarding the treatment of NLPHL derives from subgroup analyses of randomized studies comprising cHL and NLPHL patients, smaller phase 2 studies, and retrospective studies using databases from larger institutions. Although our recommendations in part differ from other guidelines, including those from the National Comprehensive Cancer Network, we believe that our approach to treating NLPHL is supported by the available data.43
Given the mostly indolent course and the excellent overall prognosis, the major goal in the treatment of NLPHL should be the optimal balance between efficacy and toxicity. Early-stage disease and intermediate-stage NLPHL should therefore be treated with 30 Gy limited-field RT alone (stage IA disease with no clinical risk factors) or a brief chemotherapy with ABVD (2 cycles for early stages other than stage IA with no clinical risk factors and 4 cycles for intermediate stages) followed by limited-field RT (20 Gy for early stages other than stage IA with no clinical risk factors and 30 Gy for intermediate stages) as these approaches are associated with an acceptable toxicity and result in long-term disease control in the majority of patients. Patients with advanced disease more often present with adverse factors such as variant histology, high lymphoma burden, and involvement of liver or bone marrow. These patients likely benefit from first-line treatment with escalated BEACOPP. However, additional analyses are necessary to define clear risk groups and confine intensive first-line treatment to high-risk patients and apply less intensive regimens in the remaining patients.
Until the required additional data are available, the efficacy and toxicity of escalated BEACOPP should be weighed against each other, and less intensive alternative approaches should also be discussed with the patient. In case the patient is not a candidate for or refuses escalated BEACOPP, R-CHOP represents the preferred protocol. The ABVD protocol is not used for the treatment of advanced NLPHL at our institution. In relapsed NLPHL, treatment should be chosen individually. Factors such as the time interval between the initial NLPHL diagnosis and disease recurrence, disease burden at relapse, affected sites at relapse, and previous therapies, as well as age and comorbidities, should be taken into account. Different treatment strategies have resulted in excellent long-term survival outcomes even in patients with repeated relapses so that overtreatment should also be avoided in this situation. Given the risk of histologic transformation into aggressive B-NHL, confirmation of the NLPHL diagnosis by biopsy is mandatory in case lymphoma recurrence is suspected. If histologic transformation into aggressive B-NHL is diagnosed, patients should be treated with R-CHOP or high-dose chemotherapy and ASCT depending on previous therapies.
Authorship
Contribution: D.A.E. and A.E. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dennis A. Eichenauer, First Department of Internal Medicine, University Hospital Cologne, Kerpener Str 62, D-50937 Cologne, Germany; e-mail: dennis.eichenauer@uk-koeln.de.