Abstract
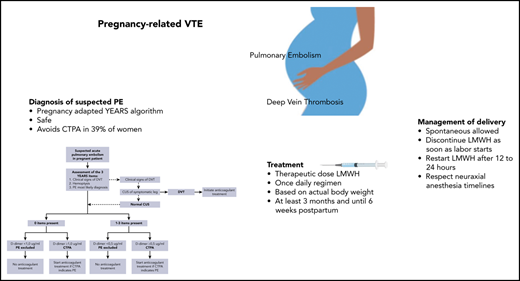
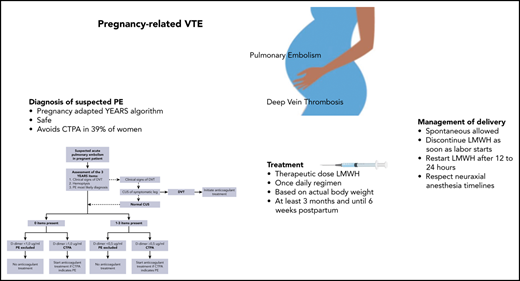
One to 2 pregnant women in 1000 will experience venous thromboembolism (VTE) during pregnancy or postpartum. Pulmonary embolism (PE) is a leading cause of maternal mortality, and deep vein thrombosis leads to maternal morbidity, with postthrombotic syndrome potentially diminishing quality of life for a woman’s lifetime. However, the evidence base for pregnancy-related VTE management remains weak. Evidence-based guideline recommendations are often extrapolated from nonpregnant women and thus weak or conditional, resulting in wide variation of practice. In women with suspected PE, the pregnancy-adapted YEARS algorithm is safe and efficient, rendering computed tomographic pulmonary angiography to rule out PE unnecessary in 39%. Low molecular weight heparin (LMWH) in therapeutic doses is the treatment of choice during pregnancy, and anticoagulation (LMWH or vitamin K antagonists [VKAs]) should be continued until 6 weeks after delivery, with a 3-month minimum total duration. LMWH or VKA use does not preclude breastfeeding. Postpartum, direct oral anticoagulants are an option if a woman does not breastfeed and long-term use is intended. Management of delivery, including type of analgesia, requires a multidisciplinary approach and depends on local preferences and patient-specific conditions. Several options are possible, including waiting for spontaneous delivery with temporary LMWH interruption. Prophylaxis for recurrent VTE prevention in subsequent pregnancies is indicated in most women with a history of VTE.
Introduction
Women who experience venous thromboembolism (VTE) during pregnancy or the postpartum period face a potentially life-threatening condition.1,2 VTE occurs in 1 to 2 of 1000 pregnancies, and the risk increases with age, mode of delivery, and presence of comorbid conditions.3-5 Almost half of women with deep vein thrombosis (DVT) in pregnancy experience a reduced quality of life as a result of postthrombotic syndrome, because thrombosis in pregnant and postpartum women predominantly affects the iliac or iliofemoral veins.6 Approximately two-thirds of lower-extremity DVTs occur antepartum, with an approximately equal distribution over the trimesters.7,8 The epidemiology of pulmonary embolism (PE) seems to differ slightly from that of DVT, with a majority of pregnancy-related episodes of PE occurring in the postpartum period.3 Given the much longer duration of the antepartum period vs the postpartum period, the daily absolute risk of VTE is highest postpartum.
A challenge in dealing with VTE issues in pregnant and postpartum women is the absence of high-quality evidence in this distinct population, although some progress has been made since the 2011 "How I treat" article9 on this topic from one of the current authors (S.M.). In the absence of evidence, it is impossible to identify optimal management, and there is wide variation among physicians, centers, and countries. In this review, we will discuss how we treat pregnancy-related VTE based on 2 patient histories from our clinical practice in an academic hospital in The Netherlands.
Case 1
A 32-year-old woman 11 weeks into her pregnancy presented to the emergency department in a teaching hospital with a 3-day history of left-sided chest pain that increased with inspiration, along with a 1-day history of shortness of breath. She had no other symptoms, most notably no fever, hemoptysis, or complaints about her legs. In the past month, she had been admitted to hospital twice because of hyperemesis and had also made a return trip to the United States (ie, 2 8-hour flights) 2 weeks before presentation. She had not received thrombosis prophylaxis. Her medical history was uneventful, except for an early miscarriage 3 years earlier; her family history was negative for VTE. On physical examination, her body weight was 78 kg with a BMI of 23.8 kg/m2. She appeared somewhat short of breath and in pain, with a respiratory rate of 22 excursions per minute, temperature of 38.2°C, blood pressure of 115/70 mmHg, regular pulse of 95 beats per minute, transcutaneous oxygen saturation of 95% on room air, and no abnormalities on chest examination. Her legs did not show signs of DVT. Laboratory results showed a normal hemoglobin level (12.6 g/dL), mild leukocytosis (11.4 × 109/L), and a D-dimer level of 10 080 ng/mL. Chest radiograph was normal. Bilateral compression ultrasonography (CUS) of the legs showed normal compressibility of the femoral and popliteal veins and no signs of flow obstruction of the iliac veins on either side. Next, computed tomography pulmonary angiography (CTPA) was performed, which showed multiple central bilateral pulmonary emboli with a normal right/left ventricle ratio (ie, no signs of right ventricular dysfunction) and a small infarction in the left lung.
Case 2
A 27-year-old woman 32 weeks into her pregnancy was transported to our emergency department because of a unilateral car accident; she had hit the guardrail of the highway. At presentation, she was unconscious, with pupils reactive on both sides, no pareses, and multiple facial injuries. Her blood pressure was 128/77 mmHg and pulse 115 beats per minute; she had a pregnant womb according to gestational age, with fetal movements observed by physical examination. Additional trauma screening revealed multiple cerebral contusional foci, intraparenchymal and subarachnoid hemorrhages, and fractures of the skull base and mastoid and sphenoid bones with pneumencephaly. Obstetric ultrasound revealed a vital fetus, without signs of placental hematoma or abruption. The present pregnancy had been uneventful except for some edema to just above the ankle on the right side for ∼2 months, without swelling of her upper leg. Two weeks before the accident, whole-leg CUS including visualization of the iliac vein had been normal. Because of the possibility that she had lost consciousness before the accident, PE was considered. On bilateral compression ultrasound, she was found to have DVT of her left femoral vein and no signs of iliac vein obstruction; on the right side, no abnormalities were seen. CT scan of the abdomen confirmed the absence of iliac vein involvement, and CTPA confirmed the diagnosis of bilateral PE, with clear signs of right ventricular dysfunction, a right/left ventricle ratio of >1, and flattening of the septum.
Diagnosis of VTE in pregnant patients
In pregnancy, specific aspects in the diagnosis of DVT and PE need to be considered. The threshold of suspicion in pregnancy is low, because VTE is a major cause of maternal morbidity and mortality. As a consequence of this, combined with the frequency of dyspnea and chest discomfort even in healthy pregnancies, the number needed to test to find a confirmed diagnosis is markedly higher than that in the nonpregnant population. Few studies have addressed the utility of an empirical clinical probability assessment or pregnancy-specific clinical decision rule, with or without the use of D-dimer levels.10,11 D-dimer levels increase during pregnancy and are often higher than the regular threshold of 500 ng/mL, thus triggering further imaging for VTE diagnosis.12 Imaging of the lungs for PE exposes both the pregnant woman and her fetus to radiation. Radiation exposure of the fetus is lowest for CTPA, whereas exposure of the proliferating breast tissue of the woman may be lower with ventilation/perfusion (V/Q) scanning.13-15 This is the reason why V/Q scanning is still the first test ordered in many North American centers, as is suggested in the American Society of Hematology (ASH) 2018 guideline.15 This guideline clearly points out that both techniques have their own risks and benefits.15 However, V/Q scanning is not widely available, and it is likely that pregnancy-adapted CTPA techniques are able to reduce the amount of maternal radiation without compromising sensitivity.
In 2018 and 2019, 2 well-sized multicenter prospective outcome studies in pregnant women with suspected PE were published, providing an evidence-based approach to this diagnostic challenge.16-18 In the first study, 395 women with suspected PE from 11 centers in France and Switzerland were included.16 PE was excluded in women with low or intermediate pretest clinical probability based on the revised Geneva score and a D-dimer level <500 ng/mL. Women with high pretest clinical probability or a D-dimer of ≥500 ng/mL underwent bilateral CUS of the legs, followed by CTPA (or V/Q scan in the case of nondiagnostic CTPA) if no DVT was found. The prevalence of VTE was 7.1%; one-quarter of these diagnoses were proximal DVTs found on ultrasound, whereas the remainder were positive CTPA results. The strategy proved to be safe, with a rate of symptomatic VTE at 3-month follow-up of 0.0% (95% confidence interval [CI], 0.0% to 1.0%) among untreated women. CTPA could be avoided in 12% based on a D-dimer of <500 ng/mL combined with low or intermediate clinical probability.
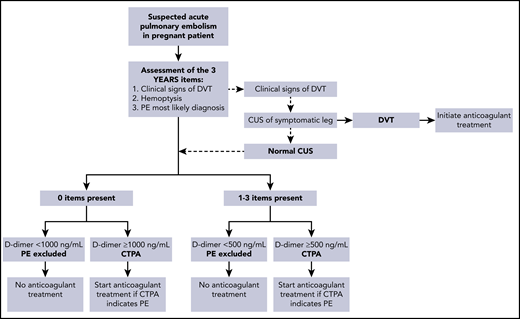
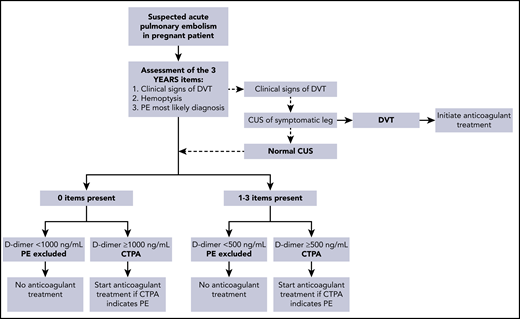
In the second study, 498 pregnant women from 18 centers in France, Ireland, and The Netherlands were managed with the pregnancy-adapted YEARS algorithm (Figure 1).17 PE was excluded in women with a D-dimer <500 ng/mL and ≥1 YEARS item (clinical signs of DVT, hemoptysis, and PE as the most likely diagnosis) or <1000 ng/mL and no YEARS items. Adaptation of the YEARS algorithm for pregnant women involved CUS, but only for women with symptoms of DVT; if the results were positive, CTPA was not performed. The prevalence of VTE was 4%; one-fifth of these diagnoses were proximal DVTs found on ultrasound, whereas the others were positive CTPA results. The strategy proved to be safe, with a rate of symptomatic VTE of 0.21% (95% CI, 0.04% to 1.20%). The upper limit of the 95% CI meets the 1.82% cutoff proposed by the International Society on Thrombosis and Haemostasis (ISTH) for confirming the safety of VTE diagnostic strategies.19 CTPA could be avoided in 39% of pregnant women. The proportions of women who were managed without CTPA were 65% in the first trimester, 46% in the second trimester, and 32% in the third trimester. On this basis, we consider the pregnancy-adapted YEARS algorithm safe and efficient, and it is the diagnostic strategy we practice in our hospital in women with suspected PE during pregnancy. Of note, in both management studies, the proportion of protocol deviations was high, indicating the challenge of using these strategies in this population. In our case 1, which occurred before publication of these management studies, the patient underwent a bilateral CUS despite the absence of leg symptoms.
Pregnancy-adapted YEARS algorithm for diagnosis of PE in pregnant women.17,18
The patient in our case 2 had had atypical leg symptoms 2 weeks before the diagnosis of PE. At that time, a formal pregnancy-adapted clinical probability assessment was not performed, but the likelihood based on clinical judgment was considered low, and DVT was considered ruled out after a negative whole-leg compression ultrasound with visualization of the iliac vein. For DVT, a pregnancy-adapted clinical prediction rule has been developed and has been retrospectively validated. In our patient, the LEFt rule (ie, symptoms in the left leg [L], calf circumference difference ≥2 cm [E for edema], and first trimester presentation [Ft]) would have been 0, indicating low clinical probability.10,20 Prospective validation of a sequential diagnostic strategy based on the assessment of clinical probability with the LEFt rule, D-dimer measurement, and complete CUS in pregnant women with suspected DVT is ongoing (registered at www.clinicaltrials.gov as #NCT01708239). The ASH 2018 guideline suggests performing serial ultrasound in women with an initial negative ultrasound, but in a prospective cohort study of 221 pregnant women assessing this strategy, all 16 DVTs were diagnosed on the first ultrasound.15,21 In another prospective study using a single whole-leg compression ultrasound to exclude DVT, the incidence of VTE during 3-month follow-up was low (1.1%; 95% CI, 0.3% to 4.0%), although the upper limit of the 95% CI does not exclude a potentially unacceptable failure rate.22 At present, we do not recommend using the LEFt rule; instead, we use a single whole-leg ultrasound with visualization of the iliac vein in women with suspected DVT.
It is important to stress that in the case of leg symptoms that suggest a pelvic vein thrombosis (mainly a swollen upper leg or pain in the buttock), and if there is uncertainty about the adequacy of ultrasonographic visualization of the iliac vein, magnetic resonance imaging may be considered as an additional test, although this technique is not validated for DVT. We will never be able to tell for sure whether our case 2 patient had DVT in her right leg that was not diagnosed, although we still deem that unlikely.
Case 1 (continued)
Our patient was treated with low molecular weight heparin (LMWH; 14 000 IU of tinzaparin once daily, based on body weight at the time of diagnosis) and admitted to the obstetric ward for 5 days for pain relief with tramadol and acetaminophen. After hospital discharge, she continued with the same dose of LMWH, to which she adhered and tolerated well apart from some mild bruising around the injection sites. She had adequate peak anti-Xa levels throughout pregnancy and normal platelet counts. Her chest pain subsided after a few weeks, but she remained somewhat short of breath throughout pregnancy. At a gestational age of 30 weeks, we performed CTPA, because she felt increasingly breathless and was tachycardic, without another clear explanation such as anemia. CTPA showed complete resolution of PE and lung infarction.
Case 2 (continued)
Our patient had probably collapsed, which led to her car accident, as a result of massive central PE with signs of right ventricular dysfunction. At presentation, she was tachycardic, but her blood pressure was adequate. Clearly, she had an absolute contraindication for both thrombolysis and therapeutic dose anticoagulation because of the neurotrauma. In the hours after admission, her cardiorespiratory status deteriorated, and after careful multidisciplinary consideration, it was decided to perform a combined procedure under general anesthesia. The interventional radiologist placed an inferior vena cava (IVC) filter through the right femoral vein below the renal veins, and the obstetrician delivered the baby through a cesarean section. After delivery, the radiologist performed thrombosuction of the pulmonary arteries with a single bolus infusion of 5000 IU of unfractionated heparin (UFH). She was then treated with a prophylactic dose of LMWH (2850 IE of nadroparin) for 6 weeks, until the intracerebral blood was fully resorbed. Unfortunately, attempts to retrieve the filter were not successful.
Treatment of VTE in pregnant patients
LMWH is the drug of choice in pregnant women, because it does not cross the placenta or have teratogenic effects.15 Table 1 lists the safety of several anticoagulants during pregnancy or breastfeeding.
Optimal use of therapeutic doses of LMWH in pregnant women is generally extrapolated from the nonpregnant population. For the initial treatment of acute VTE in pregnancy, there is no evidence base supporting a twice-daily regimen over a once-daily regimen of a therapeutic dose of LMWH.15 It is unclear whether the prepregnancy weight or the actual body weight should be used to determine the appropriate dose of LMWH, and we use the actual body weight at time of diagnosis. Although there is no evidence that anti-Xa level monitoring and subsequent dose adjustments improve clinical outcomes, there is no evidence to demonstrate these are harmful either.15 In our institution, we have the test available, and in women with acute VTE, we monitor anti- Xa levels 4 hours after injection and target to an anti-Xa level of 0.8 to 1.6 with a once-daily regimen at 6- to 8-week intervals. Practical advice is to instruct women to inject themselves in the morning, thus meeting the 4-hour postinjection time point of blood withdrawal.
The maternal safety issue of any anticoagulant is the risk of bleeding. The risk of anticoagulant-associated bleeding is thought to be low,23 because most bleeding in pregnant woman has a primary obstetric origin. A systematic review and meta-analysis that included 18 observational studies describing 981 pregnant patients using therapeutic-dose anticoagulation treatment (LMWH or UFH) for treatment of acute VTE reported an antepartum incidence of hemorrhagic complications of 3.28% (95% CI, 2.10% to 4.72%).24
LMWH leads to local bruising and skin reactions in up to 25% of pregnant patients, which are mainly type IV delayed hypersensitivity reactions at the injection site of subcutaneously administered LMWH.25,26 Type I allergy is rare and should always be considered, but if no symptoms or signs are present, we pragmatically switch to another LMWH.25,27 If all registered LMWHs lead to skin problems, danaparoid sodium or fondaparinux can be considered. A rare but serious maternal complication of heparin is heparin-induced thrombocytopenia (HIT). The incidence of HIT in pregnant patients is very low (<0.1%), but some case reports have been published.28 Although the ASH 2018 guideline on HIT suggests against platelet monitoring in patients at very low risk,29 this is a conditional recommendation, and in our institution, we monitor platelets at baseline and between 4 and 12 days postinitiation of therapy, at a time that is most practical for the patient if she is treated outside the hospital, and at infrequent intervals (6-8 weeks, coinciding with anti-Xa level and obstetric follow-up visits) thereafter. Although long-term UFH use has been associated with symptomatic osteoporosis in up to 2% of patients,30 contemporary studies of LMWH have shown that this is not an issue.31
Other anticoagulants and their use in pregnancy are either less preferred or contraindicated (Table 1). UFH can be administered both IV and subcutaneously and has a similar safety profile with regard to fetal safety but requires activated partial thromboplastin time monitoring and is associated with a higher risk of HIT.32 It is often considered for women in whom rapid reversal of the anticoagulant effect may be needed. However, these presumed benefits are offset by difficulty in maintaining therapeutic activated partial thromboplastin time results, with the obvious risk of extending thrombosis as a result of undertreatment in a woman with acute VTE and overanticoagulation in a woman in whom this option is chosen because of an increased risk of bleeding.33 In such patients, we choose a twice-daily LMWH regimen based on actual body weight over IV unfractionated heparin, with the theoretical rationale that peak anti-Xa levels are somewhat lower (at a similar area under the curve) than with a once-daily regimen34 and that in case of a bleeding emergency LMWH can still be partially neutralized by protamine sulfate.
Danaparoid also does not cross the placenta and can be used if LMWH is not an option, (eg, in the rare case of HIT).35 Fondaparinux crosses the placenta to some extent, and experience in human pregnancies, particularly during the first trimester, is limited.15,36,37 Nevertheless, we have used fondaparinux in a few women who were suspected of having type I allergy to LMWH during pregnancy. LMWH, danaparoid, and fondaparinux can be safely used in women who breastfeed.15
Because VKAs cross the placenta and may cause coumadin embryopathy and long-term effects, we avoid the use of these agents throughout the entire pregnancy for the treatment and prevention of VTE.15,38,39 VKAs can be used during breastfeeding.15
DOACs (eg, direct thrombin inhibitors and factor Xa inhibitors) are contraindicated in pregnancy and during breastfeeding.15 There is limited human safety evidence in the literature, and there seems to be animal toxicity according to the manufacturer’s summary of product characteristics.40,41 If a woman inadvertently becomes pregnant while using a DOAC, we advise switching to LMWH immediately.42 DOAC use is not regarded as medical grounds for the termination of a pregnancy.43 Physicians are recommended to report all DOAC exposure during pregnancy to the ISTH registry.44
Systemic thrombolysis should be considered in pregnant patients with PE who are hemodynamically unstable, because this condition is associated with high maternal and fetal mortality. In a literature review, 23 cases involving the use of systemic thrombolysis in pregnancy for massive PE were identified.45 There were no maternal deaths, and bleeding complications were reported in 39% of the cases. Bleeding was classified as major in 22% of women, 9% of the fetuses died, and 39% of pregnancies resulted in preterm delivery. Of course, the findings may not be accurate as a consequence of publication bias. However, the key message is that thrombolysis should not be withheld in pregnant women with life-threatening hemodynamic instability and PE, in whom risk to the fetus and risk of severe bleeding in the mother must be accepted in view of her life-threatening condition.15 Whether catheter-directed thrombolysis for severe PE is associated with a lower risk of bleeding than systematic thrombolysis is unknown, even in the nonpregnant population.46 Therefore, in our case 2 patient, we chose to perform catheter-directed thrombosuction without addition of thrombolytic agents. We generally avoid placement of an IVC filter in pregnant patients, because experience during pregnancy is limited, and filter migration and inferior caval vein perforation have been described.47 This may be disregarded in exceptional circumstances, as in our case 2 patient, in whom it was anticipated that the absolute contraindication for therapeutic anticoagulation would persist for weeks.
Case 1 (continued)
Our patient went into spontaneous labor at a gestational age of 39 + 2 weeks and delivered a healthy daughter 21 hours after the last injection of LMWH. Estimated blood loss was 200 mL, and LMWH was restarted 12 hours after delivery at full dose.
Management of delivery in women with VTE
Several options for delivery in women using anticoagulants are possible and depend strongly on local preferences and experience, which result from the perception of risks and benefits of either the wait-for-spontaneous-delivery approach or the planned-delivery approach. The ASH 2018 guideline panel suggests cessation of LMWH with spontaneous onset of labor in pregnant women receiving prophylactic-dose LMWH and planned delivery with prior discontinuation of anticoagulant therapy in pregnant women receiving therapeutic-dose LMWH.15 Of note, this conditional recommendation is based on very low certainty in evidence about effects and hence raised substantial discussion among the panel members; the pros and cons are discussed in detail in the guideline. One panelist who advocated allowing spontaneous labor with therapeutic-dose LMWH “even with the potential for limiting access to neuraxial analgesia and anesthesia and potentially increasing the risk of major bleeding”15(p3331) was one of the current authors (S.M.). In our institution, this wait-for-spontaneous-delivery approach is the default choice with all dosages. We pragmatically base this on the fact that advantages and disadvantages of both approaches are limited, and we primarily follow the golden rule of in dubio abstine (ie, when in doubt, abstain). The potential disadvantages of unplanned spontaneous delivery are the increased risk of bleeding and limiting accessibility to neuraxial analgesia. With regard to the potential increase in major postpartum bleeding, data are very conflicting and of low quality.43 In a systematic review, rates of bleeding in the postpartum period could be retrieved for 13 studies including 725 pregnancies.24 Bleeding events occurred in 38.8% of patients; major bleeding (≥1000 mL) occurred in 1.9% (95% CI, 0.80% to 3.60%), and clinically relevant nonmajor bleeding (≥500 to 1000 mL) occurred in 5.7%; the remainder were minor bleeds (<500 mL). In our own retrospective cohort study of 95 women receiving therapeutic-dose LMWH, the incidence of postpartum hemorrhage, defined as >500 mL of blood loss, was 18%, which was similar to the rate of 22% in 524 women not using anticoagulants.48 Given that the reported incidence of postpartum bleeding without anticoagulant use ranges from 4% to 22% of all pregnancies,49-51 we suspect that pregnancy-related bleeding in women using anticoagulants is generally underreported. To complicate things even further, there is no uniform definition of postpartum hemorrhage, and for anticoagulant-related bleeding events in pregnancy and the postpartum period, commonly used classification criteria may not suffice. Therefore, a proposal for classification of severity of such bleeding events was recently made by an international multidisciplinary group of clinical investigators and clinicians and also published on behalf of the ISTH subcommittee for control of anticoagulation.52
With regard to accessibility to neuraxial analgesia and anesthesia, choice of the required interval to allow neuraxial analgesia or anesthesia (ie, time intervals of 12 hours for prophylactic-dose LMWH and 24 hours for higher-dose LMWH) is very conservative and strictly followed. Alternative forms of pain relief during spontaneous labor are also available as less effective but secondary options, such as patient-controlled analgesia with remifentanil, although we acknowledge the need to pay attention to the potential for neonatal respiratory depression as a disadvantage of this method.53 For an emergency cesarean section, the only alternative is general anesthesia. Actual accessibility to neuraxial analgesia in women using LMWH seems reassuring. Preliminary results of the thrombosis prophylaxis Highlow study involving 587 women (registered at www.clinicaltrials.gov as #NCT01828697) showed that the proportions of patients using LMWH during pregnancy with a time interval that was too short to allow neuraxial anesthesia were 2.4% in patients receiving prophylactic-dose LMWH and 5.1% in patients receiving higher-dose LMWH.54 If there is no obstetric indication for an induced delivery, we instruct women to not inject LMWH as soon as labor starts with either contractions or rupture of the membranes. Active management of the third stage of labor remains necessary to minimize the risks of obstetric hemorrhage and is standard practice.
Data on the potential disadvantages of planned delivery are conflicting. Observational data have indicated consistent associations between induction of delivery and increased interventions, including cesarean section.55,56 These results are probably largely driven by indication bias. In the past decade, data of randomized trials for various indications have provided evidence to suggest that if there is a good indication for induced delivery, the increased cesarean section rate is not observed (or it can sometimes even be protective).57,58 The crucial question therefore is whether the assumed benefits of planned delivery in this setting are such a good clinical indication. These trials did not investigate other subtler outcomes that are changed by medical interventions, including the potential programming effects of late relative preterm birth, where even in the term period, subtle effects on school performance were found,59 and patient experiences.
In women at very high risk for extension or recurrent VTE (arbitrarily within 1 month before expected delivery), we consider the risks of a prolonged period without anticoagulation to be higher, and therefore, we schedule a planned delivery so that the duration of time without anticoagulation can be minimized. Those at the highest risk of recurrence (proximal DVT or PE within 2 weeks before delivery) can be switched to therapeutic IV UFH, which is then discontinued 4 hours before the expected time of delivery or the use of neuraxial anesthesia.
Case 2 (continued)
After the unsuccessful filter retrieval, therapeutic-dose LMWH was switched to full-dose DOAC. The presentation of the PE, together with the filter being present permanently as well as the patient’s preferences, led to the shared decision to continue some form of anticoagulation, and we switched to low-dose DOAC for secondary VTE prevention 4.5 months after the PE. She did not develop symptoms of postthrombotic syndrome, nor did she have residual pulmonary symptoms. She gradually recovered from her neurological trauma, and 3 years later, she had regained her old functional level and was working. Her daughter was also doing very well.
Postpartum duration of anticoagulant treatment
Anticoagulation should be restarted after delivery as soon as possible, depending on the amount of estimated vaginal blood loss and the type of delivery. Generally, restarting therapeutic-dose anticoagulation 12 to 24 hours after delivery is feasible, but this period should be longer if hemostasis is not adequate. If the anticipated interval is >24 hours because of bleeding, a prophylactic dose 24 hours after delivery should be considered. In most women in whom the intention is to stop anticoagulation 6 weeks after delivery, continuation with therapeutic-dose LMWH until 6 weeks postpartum (or until discontinuation if VTE occurred in late pregnancy) is the most practical option. In women who will continue anticoagulation indefinitely and who plan to breastfeed their babies, we first restart LMWH and initiate the first loading dose of VKAs at least 1 day later. LMWH can be discontinued after at least 3 days of VKAs and as soon as the international normalized ratio is >2.0. It is important to reassure women that they can breastfeed during use of either LMWH or VKAs, particularly nonlipophilic types such as acenocoumarol and warfarin (Table 1).15,60,61 Alternatively, if women do not plan to breastfeed, LMWH can be replaced by a DOAC. We treat women with therapeutic-dose LMWH until 6 weeks postpartum and for a minimum duration of 3 months. If the pregnancy-related VTE was the first episode, we advise discontinuation of anticoagulation after 3 months total duration or after 6 weeks postpartum. In the recent European Society of Cardiology 2019 PE guideline, pregnancy is considered a minor transient risk factor leading to an intermediate risk (3% to 8% per year) of recurrence after discontinuation of anticoagulants, with a recommendation to consider extending anticoagulation in women with pregnancy-related VTE.46
Case 1 (continued)
Two years later, the patient consulted us because she wanted to become pregnant again. Because she had a history of pregnancy-related VTE, we advised antepartum and postpartum prophylaxis with LMWH. She was enrolled in the Highlow study, an international, multicenter, randomized controlled trial comparing low-dose with intermediate-dose LWMH for the prevention of pregnancy-related recurrent VTE, and she was treated with the intermediate dose. There were no major issues during this pregnancy, and she delivered a healthy girl. She continued LMWH prophylaxis until 6 weeks after delivery.
Prevention of recurrent VTE in a subsequent pregnancy
After pregnancy-related VTE, the risk of recurrence during subsequent pregnancies is 6% to 10% if no prophylaxis is administered.62-64 The risk of recurrence is influenced by the factors present during the first VTE, as is the case for nonpregnant patients. Women who have had a single episode of VTE that was associated with a transient nonhormonal risk factor are at low risk of recurrence during pregnancy.62,63,65 For these patients, the burden of subcutaneous injections, side effects, and risk of peripartum bleeding may not outweigh the high number needed to treat during pregnancy, and only postpartum thromboprophylaxis for 6 weeks is recommended.15 In all other pregnant women with a history of VTE (ie, unprovoked VTE or VTE provoked in the presence of minor or hormonal risk factors or recurrent VTE), both antepartum prophylaxis and postpartum prophylaxis are suggested.15 Given that the increased risk of VTE is similar across trimesters, we initiate LMWH prophylaxis as soon as a pregnancy test is positive, and to do so, we prescribe a starting dose of LMWH preconceptionally. The optimal dose of LMWH for prevention of pregnancy-related recurrent VTE is unknown. Retrospective cohort studies have suggested high recurrence rates of VTE ranging from 2.5% to 8% despite thromboprophylaxis,63,64,66-69 but data are conflicting.70,71 A Cochrane review concluded that there is insufficient evidence on which to base recommendations for thromboprophylaxis during pregnancy or the early postnatal period, with the small number of differences detected in this review being largely derived from trials that were not of high methodological quality.72 Whereas the American College of Chest Physicians 2012 guideline suggests use of either a low or intermediate prophylactic dose (half of therapeutic) with no preference for 1 dose over the other,73 the 2018 ASH guideline on VTE suggests against an intermediate dose antepartum and indicates no preference for a prophylactic or intermediate dose postpartum.15 The Highlow study comparing low-dose with intermediate-dose LWMH for the prevention of pregnancy-related recurrent VTE is ongoing and will provide valuable information.74 To date, >1070 patients have been recruited, and results are expected in 2021 to 2022.
Women who use anticoagulants outside of pregnancy should, if receiving DOACs, be switched to VKAs preconceptionally.42 As soon as the pregnancy test is positive, VKAs should be switched to therapeutic-dose LMWH, and the effect of VKAs can be reversed by oral vitamin K supplements.42
Finally, it is very important that treating physicians counsel all young women with an episode of VTE about future pregnancies, as well as other related issues (ie, the 5 Ps): period, pill, prognosis, pregnancy, and postthrombotic syndrome.75
Discussion
The management of pregnancy-related VTE is based on extrapolation from the nonpregnant population, and clinical trial data on the optimal treatment are scarce. Our approach, as well as alternatives, is summarized in Table 2. LMWH in therapeutic doses is the treatment of choice during pregnancy, and anticoagulation should be continued until 6 weeks after delivery, for a minimum total duration of 3 months. Whether dosing should be based on weight or anti-Xa level is unknown, and practice differs among centers. There is limited experience with thrombolysis and IVC filter use in pregnant women, but in some cases, these interventions must be considered. Management of delivery requires a multidisciplinary approach. With some limitations, neuraxial analgesia is possible, and spontaneous vaginal delivery is our preferred option in obstetric patients who need therapeutic anticoagulation. Prevention of recurrent VTE in subsequent pregnancies is indicated in most women with a history of VTE, and results from a large randomized trial investigating the optimal dose are expected in the coming years.
Acknowledgment
The authors acknowledge K.P. van Lienden for his involvement with case 2 and critical review of the manuscript.
Authorship
Contribution: S.M. and W.G. wrote the manuscript.
Conflict-of-interest disclosure: S.M. is the principal investigator of the Highlow study and reports grants and fees paid to her institution from GlaxoSmithKline, Bristol-Myers Squibb/Pfizer, Aspen, Daiichi Sankyo, Bayer, Boehringer Ingelheim, Sanofi, and Portola. W.G. is the principal investigator of the DRIGITAT study (https://www.trialregister.nl/trial/6475) and reports an unrestricted grant from Roche Diagnostics paid to his institution for in-kind delivery of materials.
Correspondence: Saskia Middeldorp, Department of Vascular Medicine, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, Meibergdreef 9, Amsterdam, 1105 AZ, The Netherlands; e-mail: s.middeldorp@amsterdamumc.nl.