Despite significant advances in AML therapy over the past decades, 30-40% of all young patients with AML will suffer a relapse, after which achieving long-term disease-free survival remains challenging. In pediatric and young adult patients with AML, curative-intent treatment at relapse involves intensive induction therapy to achieve CR followed by consolidation with HSCT. Historically, initial response rates to salvage therapy were limited. Recently, Children's Oncology Group presented results from a Phase II study of CPX-351 (liposomal cytarabine:daunorubicin at a synergistic ratio) in pediatric patients with AML in first relapse demonstrating an impressive CR/CRp/CRi rate of 81.3%. Separately, we presented data from our first-in-pediatrics CPX-351 Phase I trial showing 48% M1 marrow response in a population consisting of children with multiply relapsed and refractory (R/R) AML patients. We also obtained single cell RNA sequencing on study patients' peripheral blasts before, during, and after CPX-351 treatment (i.e.: Days 0, 1 and 5). Preliminary data showed that CPX-351 differentially impacts p53 targets within leukemic blast clusters over time, with an enrichment for genes regulating apoptosis (e.g.: FAS, BAX) in most clusters. This suggests that these blasts may be primed for apoptosis following CPX-351 treatment. Anti-apoptosis signaling in leukemias is an established mechanism of chemoresistance. Venetoclax is an orally available, small molecule inhibitor of anti-apoptotic protein BCL-2, which is frequently overexpressed in leukemias. Based on published preclinical data and single cell RNA sequencing data from our CPX-351 trial, we developed a Phase I study to investigate the combination of CPX-351 and venetoclax in the treatment of young patients with R/R acute leukemias.
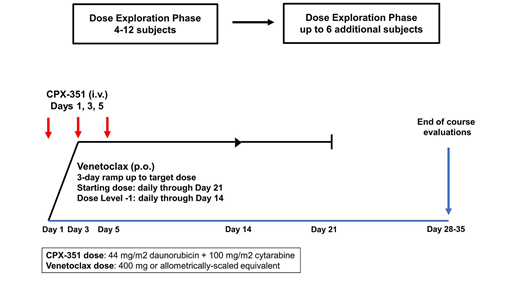
The V2 trial is a single-institution Phase I pilot study (NCT03826992) designed to test the safety and tolerability of combining venetoclax with CPX-351 in patients ages 1-39 years for the treatment of R/R acute leukemias. Inclusion diagnoses are based on preclinical sensitivities and include AML, MPAL, or AUL in first or greater relapse, or ETP-ALL, MLL-rearranged ALL, or T-ALL in second or greater relapse. Patients must have measurable disease. Exclusion criteria include CNS status 3, bone marrow failure syndromes, and prior anthracycline and mediastinal radiation exposures above acceptable cardiotoxicity risk thresholds. Subjects receive a single course of study therapy consisting of daily venetoclax and CPX-351 at the FDA approved dose for adults on Days 1, 3, and 5 (Fig. 1) with the goal of CRMRD- to allow for off-study HSCT. The venetoclax dose exploration phase begins with a starting dose of 400 mg (or the allometrically-scaled equivalent) for 21 days with a 3-day ramp to target dose. The dose exploration phase proceeds on a rolling 6 design and will require 4-12 patients to determine RP2D. Provided a tolerable RP2D is found, a dose expansion phase will open. Primary endpoints are determination of RP2D and description of the safety profile including time to hematologic recovery. Secondary endpoints are estimations of CR/CRp/CRi +/- MRD- within the context of a phase I study, and evaluation of cancer therapy-related cardiac dysfunction (CTRCD) rates. Correlative studies include evaluation of venetoclax PK with concomitant CPX-351 using modeling & simulation with a combined limited sampling and pediatric opportunistic PK sampling (POPS) strategy, and exploration of the correlations between BCL-2 family expression and pediatric tumor genetics with treatment response.
O'Brien:BTG: Research Funding; Pfizer: Research Funding; Celgene: Research Funding; AbbVie: Research Funding; Amgen: Research Funding; BMS: Research Funding. Phillips:Novartis: Membership on an entity's Board of Directors or advisory committees. Vinks:Myriad Genetics: Consultancy, Patents & Royalties. Perentesis:Kurome Therapeutics: Consultancy. Absalon:Jazz Pharmaceuticals: Other: CPX-351 for clinical trial .
This clinical trial involves the off-label use of CPX-351 and venetoclax for relapsed/refractory pediatric and young adult leukemias.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract