Background: B-cell leukemia/lymphoma-2 (BCL2), is an antiapoptotic protein commonly overexpressed in hematologic malignancies. Combination therapy with the BCL2 inhibitor venetoclax (VEN) has emerged as an efficacious treatment option for patients (pts) with hematologic malignancies. To the best of our knowledge, the outcome of VEN based therapy in MDS pts has not been reported yet.
Methods: We retrospectively reviewed MDS pts treated with VEN-based therapy between 2018-2019 including MDS/ myeloproliferative neoplasms (MPN) subtype chronic myelomonocytic leukemia (CMML) pts. Mutation testing was performed using a whole-exome next-generation sequencing panel. Clinical responders were defined by IWG2006 criteria. Pts were stratified according to the Revised International Prognostic Scoring System (IPSS-R). Overall survival (OS) was analyzed with Kaplan-Meier estimators. We analyzed the characteristics of these pts, responses to therapy, and outcomes
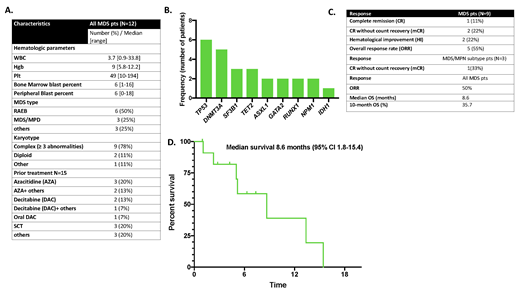
Results: Twelve MDS pts treated with VEN were identified (9 MDS and 3 MDS/MPN subtype CMML), the median follow up was 12.8 months (range, 0.3 to 40.8). Patient characteristics are shown in Figure 1A. The median age was 68 years (range, 51 to 78), 8 pts were female (66%), and 6 pts (50%) had therapy related MDS. All pts had high risk IPSS-R score (range, 6 to 10). A total of 9 (75%) pts had complex karyotype. The most common identified mutation was TP53 (Figure 1B). The median number of prior treatments was 1 (range, 1 to 3), 10 pts (83%) had relapsed/refractory (R/R) disease all of which had primary failure to hypomethylating agents (HMA), and 3 (25%) underwent allogeneic stem cell transplantation (SCT) with subsequent relapse before receiving VEN. The median dose for VEN was 200 mg (range 100 to 400mg) with median duration of 14 days (range, 7 to 21). VEN was given in combination with: 1) HMA (66%), the number of HMA doses were between 3 to 10 doses in each cycle, 2) Low dose Ara-C (8.5%), 3) Low dose Ara-C+ Cladribine+ Mylotarg (8.5%), 4) Low dose Ara-C+ Cladribine+ Ruxolitinib (8.5%), or 5) CPX-351 + Mylotarg (8.5%). The median number of cycles was 3 (range, 1 to 4). All pts had treatment delays due to slow white count recovery and 2 pts (17%) received G-CSF, 11 pts (92%) had infections and three pts (25%) died from septic shock (VEN doses were 100, and 400 mg). The overall response rate (ORR) was 55%, including one complete remission (CR) (Figure 1C). A total of 3 pts (25%) had subsequent relapse after therapy. The duration of response for pts who relapsed was 6, 8.8 and 15.6 months. Six pts (50%) transformed to acute myeloid leukemia while they were on VEN. One pt underwent allogeneic SCT after receiving VEN. With a median follow up of 12.8, the median OS was 8.6 months (Figure 1D).
Conclusion: Combination therapy with venetoclax can have activity in patients with R/R MDS but is associated with prolonged myelosuppresion. Larger studies with longer follow up are needed to determine the role of VEN-based therapy in MDS and identify potential subsets of pts who may benefit from this intervention.
Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Alvarado:Jazz Pharmaceuticals: Research Funding; Abbott: Honoraria. Kadia:Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Bioline RX: Research Funding; Celgene: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees. Ravandi:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cyclacel LTD: Research Funding; Xencor: Consultancy, Research Funding; Selvita: Research Funding; Macrogenix: Consultancy, Research Funding; Menarini Ricerche: Research Funding. Cortes:Pfizer: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Biopath Holdings: Consultancy, Honoraria; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding. Daver:Forty-Seven: Consultancy; Jazz: Consultancy; Genentech: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Glycomimetics: Research Funding; Incyte: Consultancy, Research Funding; Otsuka: Consultancy; Incyte: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; NOHLA: Research Funding; Celgene: Consultancy; Jazz: Consultancy; Servier: Research Funding; Servier: Research Funding; NOHLA: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Otsuka: Consultancy; BMS: Consultancy, Research Funding; Forty-Seven: Consultancy; Astellas: Consultancy; Agios: Consultancy; Immunogen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Astellas: Consultancy; Abbvie: Consultancy, Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Karyopharm: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Glycomimetics: Research Funding; Celgene: Consultancy; Novartis: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Agios: Consultancy; Pfizer: Consultancy, Research Funding. Takahashi:Symbio Pharmaceuticals: Consultancy. Jabbour:Cyclacel LTD: Research Funding; Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Takeda: Consultancy, Research Funding. Borthakur:FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Polaris: Research Funding; BioTheryX: Membership on an entity's Board of Directors or advisory committees; Cantargia AB: Research Funding; BMS: Research Funding; Argenx: Membership on an entity's Board of Directors or advisory committees; Oncoceutics, Inc.: Research Funding; AbbVie: Research Funding; Incyte: Research Funding; Janssen: Research Funding; GSK: Research Funding; Cyclacel: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; NKarta: Consultancy; PTC Therapeutics: Consultancy; Oncoceutics: Research Funding; Novartis: Research Funding; Xbiotech USA: Research Funding; Eisai: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Strategia Therapeutics: Research Funding; AstraZeneca: Research Funding; Agensys: Research Funding; Bayer Healthcare AG: Research Funding; Arvinas: Research Funding; Merck: Research Funding; Eli Lilly and Co.: Research Funding. Pemmaraju:incyte: Consultancy, Research Funding; affymetrix: Research Funding; sagerstrong: Research Funding; Daiichi-Sankyo: Research Funding; plexxikon: Research Funding; novartis: Consultancy, Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; cellectis: Research Funding; celgene: Consultancy, Honoraria; samus: Research Funding; abbvie: Consultancy, Honoraria, Research Funding; mustangbio: Consultancy, Research Funding. Konopleva:Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding; Amgen: Consultancy, Honoraria; Ascentage: Research Funding; Cellectis: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Eli Lilly: Research Funding; Forty-Seven: Consultancy, Honoraria; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding. Bueso-Ramos:Incyte: Consultancy. Kantarjian:Takeda: Honoraria; AbbVie: Honoraria, Research Funding; Jazz Pharma: Research Funding; Novartis: Research Funding; Ariad: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Cyclacel: Research Funding; Astex: Research Funding; Amgen: Honoraria, Research Funding; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; Pfizer: Honoraria, Research Funding; Agios: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.