TO THE EDITOR:
Factor XI is a zymogen of a serine protease that participates in the intrinsic pathway of coagulation. Deficiency of factor XI, first described in 1953,1 is characterized by mild to moderate bleeding after injury in tissues with high fibrinolytic activity such as the oral and nasal cavities and the urinary tract.2 Spontaneous bleeding is rare in factor XI–deficient patients. Treatment of factor XI deficiency is a challenge, because administration of fresh frozen plasma may lead to volume overload, and factor XI concentrates are not generally available. There are 2 plasma-derived concentrates, Hemoleven (a high-purity product from LFB Biomedicaments, France) and FXI concentrate (a partially purified product from Bioproducts Laboratory, United Kingdom), that have shown efficacy but also demonstrated safety concerns.3,4 Traces of factor XIa in the concentrates were thought to lead to thrombosis and disseminated intravascular coagulation, and current products contain heparin, antithrombin, and C1 inhibitor to eliminate factor XIa enzymatic activity. Other products have been used, such as recombinant factor VIIa with or without antifibrinolytic agents, but it is not clear which therapy is currently the best option.2 We investigated the effect of coagulation protein supplementation on thrombin generation in factor XI–depleted plasma and observed a procoagulant effect of factor IX.
Thrombin generation in platelet-poor plasma was measured with the Calibrated Automated Thrombogram (Thrombinoscope BV, Maastricht, The Netherlands) as previously described.5 Briefly, 80 µL plasma was added to 20 µL trigger reagent and incubated for 10 minutes at 37°C. Thrombin generation was initiated by adding 20 µL N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/bovine serum albumin buffer containing 100 mM CaCl2 and 2.5 mM fluorogenic substrate Z-Gly-Gly-Arg-AMC (Bachem, Bubendorf, Switzerland). Tissue factor–dependent thrombin generation was assessed with PPP Reagent low (1 pM tissue factor in the presence of 4 µM phospholipids). Fluorescence was monitored using the Fluoroskan Ascent fluorometer (ThermoLabsystems, Helsinki, Finland), and the lag time, peak thrombin, velocity index, and area under the curve were calculated using Thrombinoscope software (Thrombinoscope BV). Normal pooled plasma (obtained from more than 200 healthy individuals) or factor XI–depleted plasma (Siemens Healthcare Diagnostics, Marburg, Germany) were supplemented with prothrombin, factor X (both generously provided by the late W. Kisiel, University of Albuquerque, New Mexico, NM), factor VIII (Refacto; Pfizer, New York, NY), factor V (Haematologic Technologies, Essex Junction, VT), recombinant factor IX (Benefix; Pfizer), plasma-derived factor IX (Nonafact; Sanquin, Amsterdam, The Netherlands), activated factor IX,6 inhibitory antibodies against factor IX (IEP-5F5, prepared in our laboratory), and/or inhibitory antibodies against factor XI7 (mix of αFXI-175 and αFXI-203). Factor XI–depleted plasma obtained by immunoaffinity chromatography from large volumes of normal plasma is a commercially available substitute for patient-derived factor XI–deficient plasma.
Whole-blood thromboelastography was performed using a computerized TEG Hemostasis System 5000 (TEG; Haemonetics, Braintree, MA) according to standard protocols supplied by the manufacturer. Briefly 20 µL of 200 mM calcium chloride solution was pipetted into a TEG cup, followed by 320 µL whole-blood supplemented inhibitory antibodies against factor XI (combination of αFXI-175 and αFXI-203) and recombinant factor IX (Benefix). Coagulation was initiated with 20 µL tissue factor (final concentration 0.11 pM; Innovin), and the clotting times (R values) were determined.
Factor IXa in factor IX preparations was determined with the Rossix Factor IXa kit (Mölndal, Sweden).
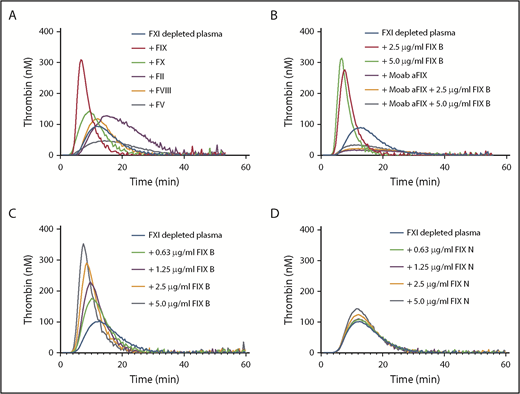
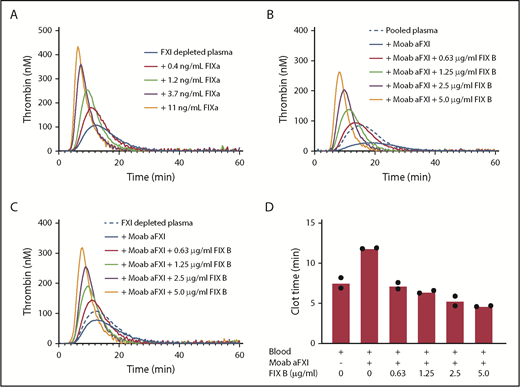
Factor XI contributes to thrombin generation when coagulation is initiated with low amounts of tissue factor.8 Therefore, thrombin generation initiated with 1 pM tissue factor was determined in factor XI–depleted plasma supplemented with downstream coagulation proteins at their respective plasma concentrations (Figure 1A). Minor procoagulant effects on thrombin generation were observed for prothrombin (increased endogenous thrombin potential) and factor X (reduced lag time). The addition of factor VIII had no effect, while factor V reduced thrombin generation. Factor V has both pro- and anticoagulant activities9 and is predominantly anticoagulant in the presence of tissue factor pathway inhibitor.10 Strikingly, a strong procoagulant effect was observed with addition of recombinant factor IX (Benefix). This enhancing factor IX effect was dependent on factor IX itself, as inhibitory anti-factor IX antibodies completely abolished the procoagulant effects of recombinant factor IX (Figure 1B). The procoagulant effect was dose dependent, as increasing concentrations of recombinant factor IX considerably enhanced thrombin generation in factor XI–depleted plasma (Figure 1C). We evaluated the effects of a commercial plasma-derived factor IX (Nonafact), but that only had a minor effect on thrombin generation in factor XI–depleted plasma (Figure 1D). A major difference between the 2 preparations is the concentration of contaminating factor IXa. Nonafact does not contain detectable amounts of factor IXa (<0.03% factor IXa, which is below the detection limit of the assay, n > 20), while Benefix contains 0.07 ± 0.02% factor IXa (mean ± standard deviation of 6 different lot numbers). Although these factor IXa concentrations in Benefix are very low, they are sufficient to enhance thrombin generation and compensate for the absence of factor XI upon activation of the extrinsic pathway (Figure 2A), Furthermore, we tested if recombinant factor IX could also improve thrombin generation in the presence of inhibiting factor XI antibodies to mimic the situation in a factor XI–deficient patient with inhibitors. The addition of anti-factor XI antibodies reduced thrombin generation in normal plasma (Figure 2B). As expected, the procoagulant effects of Benefix were not affected by anti-factor XI antibodies both in plasma containing factor XI (Figure 2B) and in factor XI–depleted plasma (Figure 2C).
Factor IX enhances thrombin generation in factor XI–depleted plasma. (A) Factor XI–depleted plasma was supplemented with 1 U/ml prothrombin (90 µg/mL), factor X (8 µg/mL), factor V (10 µg/mL), factor VIII, or factor IX (Benefix; 5 µg/ml). Thrombin generation was initiated with 1 pM tissue factor. (B) Factor XI–depleted plasma was supplemented with 2.5 or 5 µg/mL factor IX (FIX B, Benefix) in the presence or absence of a blocking anti-factor IX antibody (Moab aFIX). Thrombin generation was initiated with 1 pM tissue factor. (C) Factor XI–depleted plasma was supplemented with factor IX (FIX B, Benefix) at the indicated concentrations. Thrombin generation was initiated with 1 pM tissue factor. (D) Factor XI–depleted plasma was supplemented with factor IX (FIX N, Nonafact) at the indicated concentrations. Thrombin generation was initiated with 1 pM tissue factor. F, factor.
Factor IX enhances thrombin generation in factor XI–depleted plasma. (A) Factor XI–depleted plasma was supplemented with 1 U/ml prothrombin (90 µg/mL), factor X (8 µg/mL), factor V (10 µg/mL), factor VIII, or factor IX (Benefix; 5 µg/ml). Thrombin generation was initiated with 1 pM tissue factor. (B) Factor XI–depleted plasma was supplemented with 2.5 or 5 µg/mL factor IX (FIX B, Benefix) in the presence or absence of a blocking anti-factor IX antibody (Moab aFIX). Thrombin generation was initiated with 1 pM tissue factor. (C) Factor XI–depleted plasma was supplemented with factor IX (FIX B, Benefix) at the indicated concentrations. Thrombin generation was initiated with 1 pM tissue factor. (D) Factor XI–depleted plasma was supplemented with factor IX (FIX N, Nonafact) at the indicated concentrations. Thrombin generation was initiated with 1 pM tissue factor. F, factor.
Procoagulant effect of factor IX concentrate is caused by contamination with factor IXa. (A) Factor XI–depleted plasma was supplemented with factor IXa at the indicated concentrations. Thrombin generation was initiated with 1 pM tissue factor. (B) Normal pooled plasma was supplemented with recombinant factor IX (FIX B, Benefix) at the indicated concentrations in the presence of inhibitory anti-factor XI antibodies (Moab aFXI). Thrombin generation was initiated with 1 pM tissue factor. (C) Factor XI–depleted plasma was supplemented with recombinant factor IX (FIX B, Benefix) at the indicated concentrations in the presence of inhibitory anti-factor XI antibodies (Moab aFXI). Thrombin generation was initiated with 1 pM tissue factor. (D) Whole blood was spiked with inhibitory anti-factor XI antibodies (Moab aFXI) and recombinant factor IX (FIX B, Benefix) at the indicated concentrations. Thromboelastography was performed with 0.11 pM tissue factor, and the clot times are depicted.
Procoagulant effect of factor IX concentrate is caused by contamination with factor IXa. (A) Factor XI–depleted plasma was supplemented with factor IXa at the indicated concentrations. Thrombin generation was initiated with 1 pM tissue factor. (B) Normal pooled plasma was supplemented with recombinant factor IX (FIX B, Benefix) at the indicated concentrations in the presence of inhibitory anti-factor XI antibodies (Moab aFXI). Thrombin generation was initiated with 1 pM tissue factor. (C) Factor XI–depleted plasma was supplemented with recombinant factor IX (FIX B, Benefix) at the indicated concentrations in the presence of inhibitory anti-factor XI antibodies (Moab aFXI). Thrombin generation was initiated with 1 pM tissue factor. (D) Whole blood was spiked with inhibitory anti-factor XI antibodies (Moab aFXI) and recombinant factor IX (FIX B, Benefix) at the indicated concentrations. Thromboelastography was performed with 0.11 pM tissue factor, and the clot times are depicted.
Factor IXa has a relatively long half-life in plasma (40 minutes).11 We could confirm in our in vitro system that incubation of Benefix up to 30 minutes had no effect on its procoagulant response in factor XI–depleted plasma. Furthermore, Butenas et al have demonstrated the potential procoagulant significance of low concentrations of factor IXa in plasma and blood.12 We confirmed the procoagulant effects of Benefix in whole blood using thromboelastography with complete restoration of the clot time even at the lowest concentration of Benefix tested (Figure 2D).
Our data may have important implications for the treatment of factor XI–deficient patients. Since Benefix is a registered drug and is used in many hemophilia B patients, there is considerable evidence for its safety. Most likely, other factor IX products will also be able to improve coagulation in factor XI deficiency, but that will be dependent on their contamination with factor IXa. This can be easily determined using the methodology of our study. Furthermore, it appears that the dose of factor IX can be lower for factor XI deficiency than for factor IX deficiency, since supplementation with 0.63 µg/mL (∼0.13 U/mL) Benefix already provided considerable additional thrombin generation in factor XI–depleted plasma (Figure 1C) or whole blood spiked with anti-factor XI antibodies (Figure 2D).
Preclinical and clinical studies are urgently needed to establish whether factor IX products can be used for the treatment of bleeding in factor XI–deficient patients. In addition, since factor XI is an interesting novel target for antithrombotic therapy with low bleeding risk,13,14 the use of factor IX concentrates may be considered in those patients treated with anti-factor XI therapy who still develop bleedings.
Authorship
Contribution: K.B. designed and performed research, analyzed data, and approved the manuscript; and J.C.M.M. contributed to concept, design, and supervision of research, analyzed data, wrote the manuscript.
Conflict-of-interest disclosure: K.B. and J.C.M.M. are employed by Sanquin, the manufacturer of Nonafact. The employer of J.C.M.M. (Sanquin) received honoraria for participation in scientific advisory board panels and consulting for Bayer and Daiichi Sankyo.
Correspondence: Joost C. M. Meijers, Department of Molecular and Cellular Hemostasis, Sanquin Research, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: j.meijers@sanquin.nl.