Key Points
Germline DDX41 mutations are found in a significant proportion of sporadic MDS/AML patients.
Patients with DDX41-related MDS/AML have a relatively favorable outcome.
Abstract
Germline DDX41 mutations are involved in familial myelodysplastic syndromes (MDSs) and acute myeloid leukemias (AMLs). We analyzed the prevalence and characteristics of DDX41-related myeloid malignancies in an unselected cohort of 1385 patients with MDS or AML. Using targeted next-generation sequencing, we identified 28 different germline DDX41 variants in 43 unrelated patients, which we classified as causal (n = 21) or unknown significance (n = 7) variants. We focused on the 33 patients having causal variants, representing 2.4% of our cohort. The median age was 69 years; most patients were men (79%). Only 9 patients (27%) had a family history of hematological malignancy, and 15 (46%) had a personal history of cytopenia years before MDS/AML diagnosis. Most patients had a normal karyotype (85%), and the most frequent somatic alteration was a second DDX41 mutation (79%). High-risk DDX41 MDS/AML patients treated with intensive chemotherapy (n = 9) or azacitidine (n = 11) had an overall response rate of 100% or 73%, respectively, with a median overall survival of 5.2 years. Our study highlights that germline DDX41 mutations are relatively common in adult MDS/AML, often without known family history, arguing for systematic screening. Salient features of DDX41-related myeloid malignancies include male preponderance, frequent preexisting cytopenia, additional somatic DDX41 mutation, and relatively good outcome.
Introduction
Pathogenic germline mutations that predispose to hereditary hematological malignancies (HHMs) have been described in a growing number of genes.1 The World Health Organization 2016 classification identifies myeloid malignancies with germline predisposition as a distinct subgroup.2 Recognizing the inherited nature of myeloid disorders can be crucial in adapting clinical management, especially in the context of allogeneic hematopoietic stem cell transplantation (HSCT). Although some HHMs may be suspected based on physical congenital abnormalities, early onset, or personal or family history, others may present as sporadic.3-5 Germline mutations of the DEAD-box helicase 41 gene (DDX41) promote the development of myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) at an age similar to that observed in sporadic cohorts of both disorders.6,7 Nevertheless, the prevalence of DDX41-related malignancies and the benefit of systematic DDX41 genetic testing have not been established. Here, we screened a large, unselected cohort of adult patients diagnosed with MDS/AML to analyze the biological and clinical features of DDX41-related myeloid malignancies.
Study design
Between March 2017 and February 2019, targeted next-generation sequencing was performed on bone marrow or peripheral blood from 1385 adult patients with a diagnosis of MDS or AML in Hôpital Saint-Louis, Paris, France (supplemental Table 1; supplemental Materials, available on the Blood Web site). In aiming to identify pathogenic DDX41 germline variants, only rare variants in polymorphism databases (minor allele frequency <0.01) were retained, and a variant allele frequency (VAF) >0.4 was considered to be predictive of a germline origin (Figure 1A). Patients were subsequently referred for genetic counseling and signed an informed consent for genetic evaluation. When available, germline origin was confirmed by Sanger sequencing of cultured fibroblasts (supplemental Table 2). This study was approved by the Groupe Francophone des Myélodysplasies Institution Review Board.
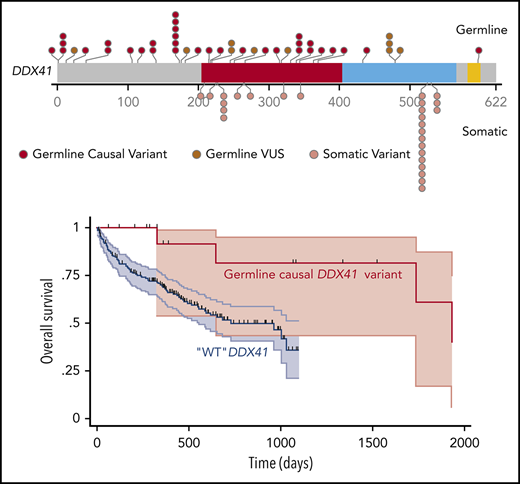
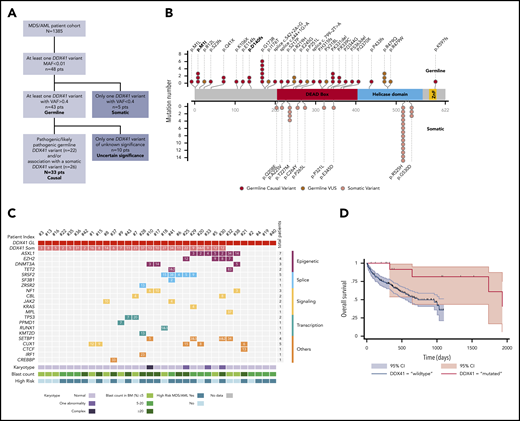
Characterization of the germline DDX41 mutated patients. (A) Flowchart of the study. (B) Graphical representation of DDX41 variants found in this study positioned on the DDX41 protein sequence, with its major functional domains. Germline variants (red) are in the upper part, and somatic variants (pink) in the lower part. Germline variants of unknown significance (VUSs) are represented in dark red. Previously reported variants are in bold. (C) Integrated matrix of the molecular, cytogenetic, and cytologic characteristics of the 33 patients with a causal germline DDX41 variant. The number reported in the box represents the VAF of each mutation. (D) Overall survival of 18 high-risk MDS/AML germline DDX41-mutated patients compared with a matched control cohort of 186 high-risk MDS/AML DDX41 wild-type patients. MAF, minor allele frequency.
Characterization of the germline DDX41 mutated patients. (A) Flowchart of the study. (B) Graphical representation of DDX41 variants found in this study positioned on the DDX41 protein sequence, with its major functional domains. Germline variants (red) are in the upper part, and somatic variants (pink) in the lower part. Germline variants of unknown significance (VUSs) are represented in dark red. Previously reported variants are in bold. (C) Integrated matrix of the molecular, cytogenetic, and cytologic characteristics of the 33 patients with a causal germline DDX41 variant. The number reported in the box represents the VAF of each mutation. (D) Overall survival of 18 high-risk MDS/AML germline DDX41-mutated patients compared with a matched control cohort of 186 high-risk MDS/AML DDX41 wild-type patients. MAF, minor allele frequency.
Results and discussion
We identified 43 (3.1%) of 1385 unrelated patients with a putative germline DDX41 variant (supplemental Table 3), 26 of whom had a second DDX41 mutation with a lower VAF (<0.4), suggestive of somatic acquisition. In all 17 patients who underwent a skin biopsy, the germline origin of DDX41 variants with a VAF ≥0.4 was confirmed. Remarkably, isolated, putatively somatic DDX41 variants were only found in 5 (0.4%) of 1385 other patients, indicating that DDX41 is rarely involved in oncogenesis in the absence of a predisposing DDX41 variant. This contrasts with other predisposing genes, such as GATA2, RUNX1, or CEBPA, which are also commonly somatically involved in sporadic MDS/AML.5
Overall, we identified 28 distinct variants, including 26 novel germline DDX41 variants (Figure 1B). Only 5 patients had the p.D140fs (n = 2) or p.M1I (n = 3) variant previously reported as the most common germline DDX41 mutations in the white population.6,7 We identified a novel recurrent variant, p.G173R, observed in 6 patients with similar biological and clinical features (supplemental Table 3), suggesting a genotype-phenotype association. We classified variants for pathogenicity according to the guidelines from the American College of Medical Genetics and Genomics8 (supplemental Table 4), and we also considered that the acquisition of a somatic DDX41 variant was a very strong criterion for causality. In 33 patients, the germline variant could be confidently considered causal, whereas in 10, it remained of undetermined significance (Figure 1A). The latter were excluded from following analyses to focus on bona fide DDX41-related myeloid malignancies, representing 2.4% of our MDS/AML cohort.
Clinical characteristics of the 33 patients are summarized in Table 1 (supplemental Table 3 summarizes individual presentation). The median age at first hematological diagnosis was 69 years (range, 36-88 years), in agreement with the prolonged latency and/or slow development of a DDX41-related malignancy and in contrast with other leukemia predisposition syndromes that arise in children or young adults.9 Most of the affected patients were men (n = 26; 79%), as reported.10 This skewing might have been due to a sex effect on penetrance, as described in other genetic disorders.11 Four patients had a concomitant solid tumor (laryngeal cancer, n = 1; breast cancer, n = 1; and prostate cancer, n = 2). Strikingly, 3 patients (9%) had a personal history of lymphoma (Hodgkin disease, n = 1; non-Hodgkin lymphoma, n = 2), suggesting that DDX41 variants may also predispose to lymphoid neoplasms, in agreement with a previous report.7 Only 9 patients (27%) had a familial history of hematological malignancy (pedigrees shown in supplemental Figure 1), underlining the rationale for systematic DDX41 genetic testing in MDS/AML, irrespective of family history.
At first diagnosis of myeloid disorder, according to the World Health Organization classification,2 4 patients had MDS with multilineage dysplasia, 11 had MDS with excess of blasts, 11 had AML (M2, M1, and M0; no M612 ), 3 had MDS/myeloproliferative neoplasm, 3 had aplastic anemia, and 1 had isolated neutropenia (Table 1). Importantly, 15 patients (46%) had a previous history of cytopenia, starting a median of 5.2 years (range, 1.8-15.2 years) before diagnosis of myeloid malignancy. This contrasts with the initial report from Lewinsohn et al,7 where germline DDX41 mutation carriers had normal blood counts until overt myeloid malignancy. However, that study focused on families with multiple cases of MDS/AML, and patients were younger at diagnosis (median, 57 years) compared with our cohort. Most of our patients had a normal karyotype (85%), which differs from sporadic MDS, where an abnormal karyotype is observed in half of the patients.13 The most frequent somatic alteration was a second DDX41 mutation, found in 26 (79%) of 33 patients (Figure 1C; supplemental Table 5). It was previously shown that this DDX41 somatic hit involved the remaining wild-type allele.6 Of note, in some patients, this mutation was present at a low VAF, suggesting that it may not always be the major driver of oncogenesis. In addition, 67% of patients had at least 1 oncogenic mutation (range, 0-5 mutations) in various genes recurrently involved in myeloid neoplasms, including splice factor mutations (although these were previously reported to be mutually exclusive to DDX41 mutations).6 A recent study reported a somatic TP53 mutation in one-third of DDX41-mutated patients, whereas this was marginal at 6% in our cohort. However, as acknowledged by the authors, their cohort mainly included patients with high-grade neoplasms, in contrast with our cohort.10
With a median follow-up of 2.8 years, the median OS was not reached, and the 2-year survival was 90% (supplemental Figure 2). Nine high-risk MDS/AML patients received intensive chemotherapy; all achieved a complete response, and 4 were bridged to HSCT. Eleven high-risk MDS/AML patients received azacitidine; 5 of them achieved a CR, and 3 had hematological improvement, leading to an overall response rate of 73%, associated with a prolonged duration of response (median, 2.5 years; range, 1.3-4 years). Seven patients underwent HSCT without unexpected toxicity. To specifically evaluate the impact of DDX41 mutation on survival among patients treated at our institution, we conducted a propensity score–matched comparison of patients with high-risk MDS/AML treated with azacitidine or intensive chemotherapy (supplemental Methods). The median OS of the 18 matched patients with DDX41 mutations was longer as compared with that of the 186 DDX41 wild-type matched controls (5.2 vs 2.7 years; Figure 1D), without reaching statistical significance. Overall, DDX41-related HHMs seemed to have favorable outcomes in our cohort when compared with sporadic MDS/AML in elderly patients.14,15 This is in contrast with the study from Polprasert et al,6 which reported a shorter OS of DDX41-related MDS/AML patients. However, this cohort included not only germline DDX41-mutated patients but also patients with somatic deletion of DDX41 as a consequence of 5q deletion, which might explain differences in outcome.
For some patients in our cohort, the identification of a germline DDX41 mutation had a direct consequence on clinical management. Indeed, because HSCT from DDX41 mutation carriers may promote donor cell leukemias,16,17 we excluded DDX41 mutation carriers as potential related donors for our patients. Regarding genetic counseling and follow-up of asymptomatic carriers, there are to date no consensus recommendations. However, in our experience, DDX41-mutated patients frequently present with mild cytopenia years before overt hematological myeloid malignancy, suggesting that watchful surveillance would allow the detection of disease evolution. Longitudinal studies of large cohorts are warranted to establish the natural history of DDX41-related HHMs, to determine the penetrance, including sex effect and lymphoid malignancies, and finally to refine clinical management and genetic counseling.
In conclusion, this is the first study identifying DDX41-related myeloid malignancies within a large, unselected cohort of patients with MDS/AML. The frequency of 2.4% we observed compares with the estimated overall HHM prevalence of 5% in adult patients with MDS/AML.18,19 This makes DDX41 the most common predisposition gene for myeloid malignancies in adults, while most patients have no family history. We report distinct biological features and favorable outcomes, which define DDX41-related myeloid malignancies as a specific entity. Our study highlights the importance of systematic testing for DDX41 mutations in adult MDS/AML, because identification of this condition may inform clinical management.
Myeloid panel gene sequencing data are available by request to the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.S., L.A., and E.C. designed the study; M.P., A.R., S.Q., N.V., M.D.C., J.S., and E.C. provided and analyzed biological data; M.S., R.R., E.R., F.S.d.F., M.C., N.B., H.D., R.P.d.L., G.S., R.I., P.F., and L.A. managed patients and provided clinical data; M.S., M.P., J.S., L.A., and E.C. wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marie Sébert, Service Hématologie Sénior, INSERM/CNRS UMR 944/7212, Hôpital Saint-Louis, 1 av Claude Vellefaux, 75010 Paris, France; e-mail: marie.sebert@aphp.fr.
REFERENCES
Author notes
L.A. and E.C. contributed equally to this work.