Abstract
Recent multistate outbreaks of coagulopathy caused by brodifacoum-tainted synthetic cannabinoids or “fake weed” highlight the public health impact of long-acting anticoagulant rodenticides (LAARs). Patients presenting with this syndrome have had recent exposure to synthetic cannabinoids, evidence of isolated vitamin K antagonism with or without bleeding, and detectable levels of brodifacoum and other LAARs in circulation. This article will provide information on synthetic cannabinoids, LAARs, and coagulopathic manifestations arising from use of adulterated synthetic cannabinoids and their management.
Introduction
In March 2018, the Illinois Department of Public Health (IDPH) was informed by the state poison center of 4 patients presenting to local emergency departments over a 2-week period with unexplained bleeding and markedly elevated international normalized ratios (INRs; 5 to >20; normal INR, <1.1).1 None of the patients reported accidental exposure to rodenticides or receipt of anticoagulants. Further investigation by the IDPH revealed that all patients reported synthetic cannabinoid use in the previous 3 days. In the ensuing months, an additional 155 cases related to this outbreak were identified. In a limited number of cases where plant material was available, testing revealed adulteration with brodifacoum,1 a long-acting anticoagulant rodenticide (LAAR) frequently implicated in accidental poisonings.2,3 Between 1 March 2018 and October 26 2018, the Centers for Disease Control and Prevention received reports of >300 similar cases, including 7 deaths, from state health departments across the United States, with the largest cluster of cases being reported in Illinois (Johnni Daniel and Luke Yip, Centers for Disease Control, e-mail, 9 November 2018).
Synthetic cannabinoids
Synthetic cannabinoids are a large group of heterogeneous chemical compounds that functionally resemble Δ9-tetrahydrocannabinol (THC), the active ingredient in marijuana. THC exerts its psychoactive effects through binding the cannabinoid receptors (CB1 and CB2), G-protein–coupled receptors that are abundantly expressed in the brain (CB1)4 or the periphery (CB1 and CB2). CB1 activation inhibits neurotransmitter release from nerve terminals, whereas activation of CB2 receptors, primarily expressed on immune cells, induces apoptosis and inhibits cytokine release.5,6
Since the discovery of THC as the active ingredient in marijuana,7 pharmaceutical companies have shown interest in developing synthetic THC analogs for therapeutic use. Although THC analogs, such as dronabinol, the active ingredient in Marinol and Syndros, have been commercially developed, most have not been developed for clinical use because of undesirable effects on the central nervous system.6 However, these synthetic compounds have been attractive to the illicit drug market because of their small size (20-26 carbons), high potency, and ease of synthesis.8 Since 2004, these compounds, either mixed with or sprayed onto dried plants, have been marketed as incense blends or aromatherapy under various brand names (eg, K2 or Spice) and sold at gas stations and convenience stores and on the Internet.9
Unlike THC, which is only a partial agonist of the CB1 receptor, some synthetic compounds exert full agonist activity, accounting for adverse reactions ranging from tachycardia, hypertension, and agitation to life-threatening complications including acute kidney failure, myocardial infarction, seizures, and, in severe cases, death. A recent description of a “zombie outbreak” resulting from mass intoxication of 18 individuals with a highly potent synthetic cannabinoid was reported in New York.10 Because of increasing reports of such adverse reactions, the US Drug Enforcement Agency and several European nations have regulated and/or banned the sale of these compounds. However, manufacturers have become adept at circumventing regulations by altering their chemical composition and/or through evasive labeling (eg, “not for human consumption”).
LAARs
To date, all reported cases of cannabinoid-associated coagulopathy, where vitamin K antagonist (VKA) adulteration was suspected, have identified contamination with brodifacoum with or without other VKAs. The rationale for mixing brodifacoum with synthetic cannabinoids remains currently unknown. It has been speculated, however, that adulteration with brodifacoum, a highly lipophilic agent, either prolongs the high from synthetic cannabinoids by interfering with its metabolism or directly potentiates the drug effect. Isolated instances of brodifacoum-tainted cocaine and marijuana for the intended purpose of enhancing the drug effect have been reported.11,12
Brodifacoum, the active ingredient in the commercial rodenticide d-CON, was developed in 1975 to overcome warfarin resistance in the rodent population.13 Brodifacoum, warfarin, and related compounds interfere with γ-carboxylation of glutamic acid residues required for vitamin K–dependent proteins, including several coagulation factors (factors VII, IX, X, and II), anticoagulant proteins (proteins C, S, and Z), and proteins involved in bone and soft tissue mineralization (osteocalcin and matrix gla-protein).14
Of the LAARs, brodifacoum is the most widely used and most commonly associated with accidental or intentional poisoning.3 For a comprehensive review on the epidemiology of LAAR poisoning, the reader is referred to the recent review by King and Tran.3 Exposure to LAARs can occur through different routes, including ingestion, inhalation, and transcutaneous exposure.3 Large-scale poisoning attempts with brodifacoum15 have also raised serious public health concerns surrounding intentional misuse. Because of the commercial availability of brodifacoum, the variety of routes with which individuals can be exposed to drug, and the delayed manifestations of symptoms after poisoning, the US government lists brodifacoum on the chemical threat risk assessment compound list.15
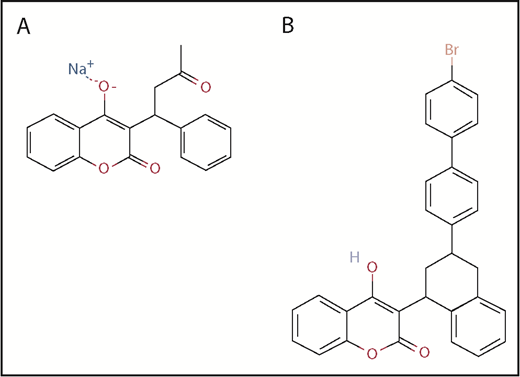
Brodifacoum consists of a substituted phenyl chain for the terminal methyl group of warfarin, which accounts for its high affinity for vitamin K epoxide reductase, resulting in a 40- to 50-fold increase in hepatic concentration and 100-fold increase in potency relative to warfarin (Figure 1).16-18 Pharmacokinetic studies in rabbits19 and poisoning cases in humans20 indicate a high volume of distribution and a biexponential decay caused by slow plasma clearance. In humans, the terminal half-life of brodifacoum is estimated to be ∼16 to 36 days, as compared with warfarin, with a plasma half-life of ∼17 to 48 hours depending on dose (Table 1).20-22 Serum brodifacoum levels can be quantified using high-performance liquid chromatography23 and seem to correlate with anticoagulant activity. In poisoning cases where serial brodifacoum levels were monitored in patients receiving vitamin K therapy, brodifacoum levels <10 ng/mL were associated with a normal coagulation profile and allowed for safe discontinuation of vitamin K1.20,24,25
Two-dimensional chemical structures of warfarin and brodifacoum. Structures of warfarin (A), a 4-hydroxycoumarin, and brodifacoum (B), which also carries a 4-hydroxycoumarin ring system like warfarin but has a substituted tetrahydronaphthyl side chain in the terminal methyl group. Adapted from the National Center for Biotechnology Information, PubChem Compound Database.40,41
Two-dimensional chemical structures of warfarin and brodifacoum. Structures of warfarin (A), a 4-hydroxycoumarin, and brodifacoum (B), which also carries a 4-hydroxycoumarin ring system like warfarin but has a substituted tetrahydronaphthyl side chain in the terminal methyl group. Adapted from the National Center for Biotechnology Information, PubChem Compound Database.40,41
Clinical features of cannabinoid-associated coagulopathy
The demographics and clinical features of patients with cannabinoid-associated coagulopathy have recently been described.1,26 The case series by Moritz et al1 describes 155 patients (76 confirmed and 79 probable) evaluated by the IDPH; Kelkar et al26 describe a subset of this larger cohort (n = 34), who presented to 1 medical center and underwent additional evaluation. In the IDPH series, patients presenting with coagulopathy were young (median age, 32 years; range, 18-65 years) and predominantly men (74%) and white (52%). Consistent with epidemiologic data on VKA poisoning,3 major bleeding symptoms included gross hematuria and other signs of mucocutaneous bleeding (eg, bruising, melena, hematemesis, and expistaxis). Nonbleeding symptoms reported by Kelkar et al26 included a high incidence of abdominal (47%) and flank pain (38%), associated with radiographic abnormalities of the renal and urothelial collecting systems in ∼50% of symptomatic patients. Intracranial bleeding was reported in 2.6% to 2.9% of patients and contributed to mortality in all cases.1,26 INR was prolonged in all patients, with a mean INR of 15 in the report by Kelkar et al.26 Circulating brodifacoum levels were detected in all patients who were specifically tested for drug. Additional VKAs were detected in some patients, including difenacoum, bromadiolone, and warfarin.26
The majority of patients interviewed reported using synthetic cannabinoids once per day, or more often, and described use of multiple illicit products. In the report by Kelkar et al,26 the time from inhalation to symptoms was short (1-3 days). No uniform source of product was identified by the IDPH.
Diagnosis
Given the increasing use of synthetic cannabinoids, patients exposed to these agents should have prothrombin time (PT) checked to identify asymptomatic coagulopathy. Because the amount of brodifacoum in plant material and the amount of brodifacoum absorbed after each use of synthetic cannabinoid are variable, time to PT prolongation after synthetic cannabinoid exposure is unknown. Laboratory confirmation of suspected brodifacoum poisoning requires demonstration of decreased levels of vitamin K–dependent coagulation factors, exclusion of other types of coagulopathy, including liver disease and/or disseminated intravascular coagulation (through measurement of liver function tests and fibrinogen, D-dimer, and factor V levels), and detection of circulating anticoagulants by liquid chromatography and mass spectrometry. Presently, there are 2 laboratories that provide qualitative or quantitative testing for brodifacoum levels: NMS Labs (Anticoagulant Poisoning Panel, catalog #0406B; Willow Grove, PA), which offers commercial testing, and the Wisconsin State Laboratory of Hygiene, which provides quantitative brodifacoum testing upon request. The Anticoagulant Poisoning Panel also analyzes for other LAARs as well as warfarin. Cases related to cannabinoid use should be reported to the local poison control center as well as local or state health departments.
Treatment
As with any VKA, initial management of brodifacoum toxicity is directed to controlling any bleeding symptoms and correcting the coagulopathy. For major bleeding symptoms, reversal with a 4-factor prothrombin complex concentrate (Kcentra or Beriplex) or, if this is unavailable, fresh frozen plasma should be initiated, along with administration of vitamin K1 (phylloquinone/phytonadione). Presently, there is no consensus on the optimal initial dosing of vitamin K1, with widely ranging doses (10-600 mg per day) reported in the literature.3 Generally, the lowest dose of vitamin K1 required to normalize PT and INR to normal or near-normal levels is advised. IV vitamin K1 achieves rapid correction of INR (within 6-12 hours) as compared with oral vitamin K1 and should be administered to patients with active bleeding. Incidence of anaphylactic reactions with IV vitamin K1 is low and estimated to be 0.03%27 and is thought to be caused by the polyethoxylated castor oil in the IV formulation.3 Frequent dosing (3-4 times a day) is recommended for both IV and oral vitamin K1 because of the short half-life (∼1-2 hours).28,29 Therapeutic plasma exchange has been used in a single patient with brodifacoum intoxication and anaphylactoid reactions to parenteral vitamin K1,30 but the efficacy of this approach with a drug having a high volume of distribution and long half-life is unknown.31 For additional information regarding the management of LAAR poisoining, the reader is referred to the recent review by Schulman and Furie.32
Given the long half-life of brodifacoum and the other LAARs, vitamin K1 supplementation needs to be maintained for an extended period of time. In 1 review, the median duration of treatment was 140 days, with a range extending to 730 days.3 In general, PT/INR are sufficiently sensitive for monitoring the need for continued vitamin K1 therapy. PT/INR should be measured within 48 to 72 hours after vitamin K1 discontinuation to confirm that they remain normal. For patients who may have other causes of PT/INR prolongation (eg, dietary vitamin K deficiency or underlying liver disease), measurement of brodifacoum levels may be useful. In a case report of brodifacoum poisoning where serial drug levels were obtained, elimination kinetics of brodifacoum appeared linear and were useful for predicting duration of vitamin K1 support.24 Other reports have suggested that vitamin K1 can be discontinued without rebound coagulopathy at levels of 4 to 10 ng/mL.20,24,25
The need for an extended outpatient course of therapy with oral vitamin K1 presents a problem for many patients because of the high cost of oral therapy. A 1-month supply of phytonadione at a dose of 100 mg daily costs ∼$25 000 to $37 000.26,33,34 Oral administration of liquid vitamin K1, which is less expensive, has been used in some cases.34,35 Phenobarbital, an inducer of the cytochrome P450 system, accelerates warfarin metabolism36,37 and has been used in some reports as adjunctive treatment of brodifacoum poisoning.25,38,39 Reports of phenobarbital dosing for this indication in adults range from 6039 to 120 mg per day in divided doses38 ; doses of 2 mg/kg were used in a pediatric case.25 It should be noted, however, that the effectiveness and risks/benefits of phenobarbital for LAAR reverse are currently unknown.
Conclusion
Cannabinoid-induced coagulopathy is caused by adulteration of synthetic cannabinoids or “fake weed” with LAARs, most commonly brodifacoum. Affected patients are often young, have no antecedent anticoagulation use or rodenticide exposure, and may admit to recent inhalation or ingestion of synthetic marijuana. Clinical manifestations range from asymptomatic laboratory coagulopathy to severe life-threatening bleeding. Synthetic cannabinoids cannot be detected with routine urine drug tests. Laboratory evaluation requires documenting deficiency of vitamin K–dependent factors and, if possible, detection of LAARs through specialized testing. Severe coagulopathy, with or without bleeding, should be promptly reversed with the use of 4-factor prothrombin complex concentrates and IV vitamin K1. Because of prolonged effects of brodifacoum, treatment requires extended monitoring of INR and prolonged treatment with oral or subcutaneous vitamin K1 therapy until circulating drug levels become undetectable. Monitoring of brodifacoum levels may guide duration of vitamin K1 therapy.
Acknowledgments
The authors thank Luke Yip and Erin Morwitz for their critical review of and feedback on the manuscript.
Authorship
Contribution: G.M.A. and T.L.O. jointly wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gowthami M. Arepally, Division of Hematology, DUMC Box 3486, Rm 356, A Alex H. Sands Bldg, Research Dr, Durham, NC 27710; e-mail: arepa001@mc.duke.edu.