Key Points
MRD using NGS-identified patients with an excellent outcome in multiple myeloma.
MRD should be assessed in every prospective trial, and is a candidate to become a primary end point.
Abstract
The introduction of novel agents has led to major improvements in clinical outcomes for patients with multiple myeloma. To shorten evaluation times for new treatments, health agencies are currently examining minimal residual disease (MRD) as a surrogate end point in clinical trials. We assessed the prognostic value of MRD, measured during maintenance therapy by next-generation sequencing (NGS). MRD negativity was defined as the absence of tumor plasma cell within 1 000 000 bone marrow cells (<10−6). Data were analyzed from a recent clinical trial that evaluated the role of transplantation in newly diagnosed myeloma patients treated with lenalidomide, bortezomib, and dexamethasone (RVD). MRD negativity was achieved at least once during maintenance in 127 patients (25%). At the start of maintenance therapy, MRD was a strong prognostic factor for both progression-free survival (adjusted hazard ratio, 0.22; 95% confidence interval, 0.15-0.34; P < .001) and overall survival (adjusted hazard ratio, 0.24; 95% confidence interval, 0.11-0.54; P = .001). Patients who were MRD negative had a higher probability of prolonged progression-free survival than patients with detectable residual disease, regardless of treatment group (RVD vs transplant), cytogenetic risk profile, or International Staging System disease stage at diagnosis. These results were similar after completion of maintenance therapy. Our findings confirm the value of MRD status, as determined by NGS, as a prognostic biomarker in multiple myeloma, and suggest that this approach could be used to adapt treatment strategies in future clinical trials.
Introduction
Multiple myeloma is a hematologic malignancy characterized by the accumulation of malignant plasma cells, usually within the bone marrow.1 This disease has long been considered incurable, supporting prolongation of overall survival as the major objective in both clinical practice and prospective trials. The introduction of several novel drugs over the past decade has dramatically improved patient outcomes, extending survival from 3 years to >10 years in young, transplant-eligible patients.2-5 As a result, overall survival has become an unrealistic objective for clinical trials, and most investigators, as well as health agencies, now focus on alternative end points, such as progression-free survival or complete remission rates. Although these 2 end points may be more realistic, in terms of the time required to answer the question, they are far from perfect. Furthermore, with the current highly effective drug combinations, complete response (CR) rates have increased dramatically from a few percent to up to 80%,6 making this particular end point less useful. A solution to improve upon CR is to use more sensitive response assessment techniques, enabling quantification of the so-called “minimal residual disease” (MRD). This approach has been successfully used in several hematological malignancies such as acute lymphoblastic leukemia,7 acute promyelocytic leukemia,8 or chronic lymphocytic leukemia.9 Number of pilot studies have shown benefit of achieving MRD negativity using currently available methods in myeloma: next-generation flow (NGF),10-12 and next-generation sequencing (NGS).13 Each of these techniques is capable of detecting a single residual malignant plasma cell within 1 000 000 bone marrow cells.
Using data from the recent phase 3, Intergroupe Francophone du Myélome (IFM) 2009 clinical trial, which evaluated the role of transplantation in patients with newly diagnosed myeloma treated with lenalidomide, bortezomib, and dexamethasone (RVD),11 we assessed the prognostic value of MRD measured during maintenance by NGS.
Patients and methods
Patients
The protocol and clinical results of the IFM 2009 trial, including MRD results obtained by multiparametric flow cytometry, have been previously described in detail.14 Briefly, 700 patients were enrolled in France, Belgium, and Switzerland between 2010 and 2012. The trial was approved by local and national health authorities, and all patients signed an informed consent form for MRD analyses. Participants were transplant-eligible patients younger than 66 years of age, with newly diagnosed, symptomatic multiple myeloma. Patients were randomized to receive either a conventional-dose strategy (ie, 8 courses of the RVD regimen) or an intensive approach comprising 3 courses of RVD followed by high-dose melphalan (200 mg/m2) with autologous stem cell transplantation, and consolidation with a further 2 cycles of RVD. All patients received lenalidomide maintenance therapy for 12 months. Randomization was stratified according to International Staging System (ISS) disease stage15 and cytogenetic risk profile, with high-risk patients defined at diagnosis by the presence of 17p deletion or either t(4;14) or t(14;16) translocation. When this trial was designed in 2008, the NGS technique was not available; therefore, MRD was assessed by low-sensitivity (10−4; ie, 1 malignant plasma cell within 10 000 bone marrow cells) multiparametric flow cytometry in all patients who achieved at least a very good partial response (VGPR) following consolidation. Bone marrow samples were collected from all such patients at both the start and end of maintenance therapy (the MRD time points), for the measurement of MRD.
MRD assessment
Details of the multiparametric flow cytometry technique have already been published.16 Collected bone marrow samples were frozen as dry pellets and stored at −80°C until analysis. When NGS commercial kits became available (Sequenta, San Francisco, CA),13 DNA was extracted from the stored bone marrow samples and sent for sequencing. Briefly, the technique is based on the sequencing of immunoglobulin genes, which are clonally rearranged in all myeloma patients and thus represent unique, patient-specific biomarkers. As the technique required prior identification of the clonal rearrangements, tumor DNA obtained from CD138+ cells at enrollment were sent to Sequenta for assessment. The clonal rearrangements were identified using the NGS MRD assay (Sequenta). For MRD quantification, DNA was extracted from the bone marrow samples and amplified by polymerase chain reaction using immunoglobulin gene-specific primers; the amplified products were then sequenced. The sensitivity was 10−6, that is, 1 malignant plasma cell within 1 000 000 bone marrow cells. MRD levels were reported as follows: <10−6, from 10−6 to <10−5, from 10−5 to <10−4, and 10−4 or greater. Patients who failed to achieve at least a VGPR, or who did not enter the maintenance phase of the trial, were considered MRD+. Based on the results described in “Patients and NGS MRD assessments” (supplemental Table 1, available on the Blood Web site; supplemental Figure 1A-B), patients were considered MRD− when the level was below 10−6.
Statistical analyses
We assessed the association between MRD status during maintenance therapy and survival end points using 2 populations defined at the start, and after the completion, of 12 months of maintenance therapy (landmark times): (1) including only patients in whom MRD status was known at this time and (2) a modified intent-to-treat population that only excluded patients who had achieved VGPR, CR, or stringent complete remission at both the start and end of maintenance therapy, but in whom MRD was not measured at either time point. Progression-free survival was defined as the time from randomization or from the landmark points until either the first documentation of progressive disease or death from any cause. Overall survival was defined as the time from randomization or from the landmark points until death. The data were updated in February 2017, and follow-up was estimated using the reverse Kaplan-Meier method.4 Survival functions were compared using the log-rank test or the Mantel-Byar test,17 and graphed using the Kaplan-Meier method or the Kaplan-Meier method modified by the Simon-Makuch method18 in the landmark analyses and the modified intent-to-treat analyses, respectively. The prognostic value of MRD was evaluated using a multivariate Cox proportional hazards model including MRD as a fixed covariate or as a time-dependent covariate. These models were adjusted for stratification factors and treatment groups. Analyses of progression-free survival in specific subgroups were performed entering an interaction term between subgroup and MRD status in the Cox models. The proportionality assumptions were checked with Cox-Snell residuals. All models were repeated using imputed values for missing assessments. MRD negativity rates were compared between groups using the χ2 or Fisher exact test. A logistic model adjusted for stratification factors was used to determine the association between treatment group and MRD status. Tests were 2 sided, and P < .05 was considered significant. Given the exploratory nature of this post-hoc analysis of the IFM 2009 study, no adjustment was made for multiple testing. All analyses were conducted using Stata Version 14.2 (StataCorp LP, College Station, TX).
Results
Patients and NGS MRD assessments
MRD status was assessed in 224 of 366 patients at the start and 183 of 239 patients after completing maintenance therapy, as they achieved at least a VGPR and were included in the analyses. In all, 138 patients had MRD status assessed twice (supplemental Figure 2). The reasons for missing assessments in patients who achieved at least a VGPR are summarized in supplemental Table 2. We also performed a modified intent-to-treat analysis which excluded 191 patients who achieved at least a VGPR at both the start and end of maintenance therapy, but in whom MRD was not assessed at either time point. Among the 509 remaining patients, 269 in VGPR were assessed at least once during maintenance therapy. Baseline characteristics were comparable between patients with known and unknown MRD status, and between treatment arms both at the landmark time points and overall (supplemental Tables 3-4). Initially evaluating the depth of MRD, we found progression-free survival to be related to the level of MRD at both time points, with the best outcome observed when MRD level was below 10−6 (supplemental Table 1; supplemental Figure 1A-B). This level was hence considered as MRD−.
MRD status by treatment arm, stratification factors, and response rates in the modified intent-to-treat population
Table 1 summarizes the MRD results by treatment arm, stratification factors, and treatment response. In total, 127 patients (25%) achieved MRD− status at least once during maintenance: 54 of 264 patients (20%) in the RVD-alone arm, and 73 of 245 patients (30%) in the transplantation arm (adjusted odds ratio for undetectable MRD 1.65; 95% confidence interval, 1.10-2.49; P = .02). The MRD negativity rate did not differ according to ISS disease stage or cytogenetic risk profile (high vs standard risk). Among patients with high-risk cytogenetics, MRD negativity was achieved in 17 of 42 patients with t(4;14) (40%), but in only 3 of 28 patients with del(17p) (11%). Among patients who started maintenance therapy, 31% and 49% of patients who achieved a VGPR and a complete response, respectively, were MRD− (P = .006). Of those 233 patients who had previously been found to be MRD− by multiparametric flow cytometry, 120 (52%) were confirmed as MRD− by NGS. Change in MRD status before and after maintenance therapy is reported in supplemental Table 5.
Progression-free survival
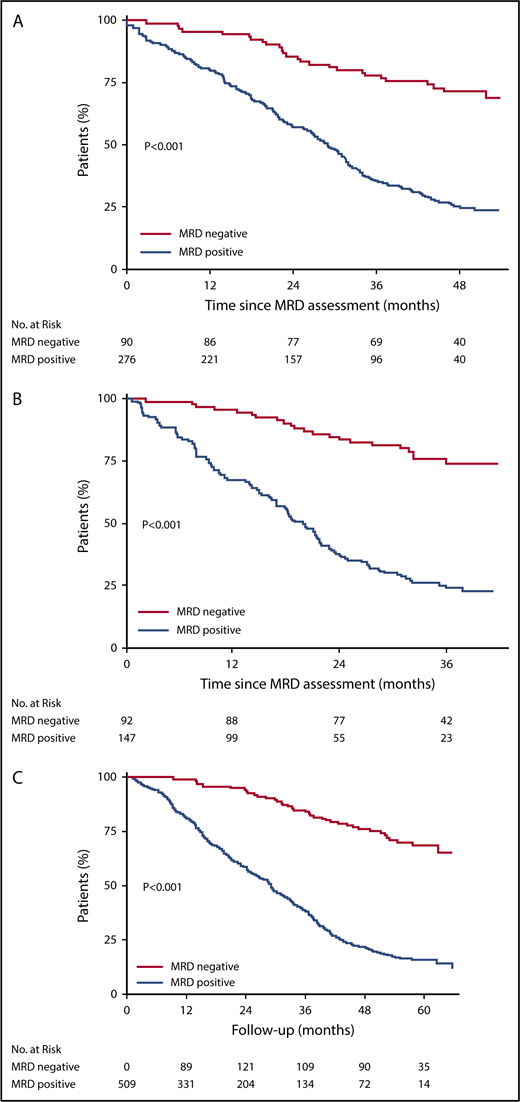
The median duration of follow-up was 55, 50, and 38 months from randomization, start, and completion of maintenance therapy, respectively. Progression-free survival was significantly prolonged in MRD− vs MRD+ patients (Figure 1). A similar significant difference is also observed using landmark and modified intent-to-treat population (supplemental Figure 6). Among the 37 MRD− patients who progressed or died during follow-up, 34 had a serological relapse. The progression-free survival benefit associated with MRD negativity was similar among the different patient subgroups (supplemental Figure 3). In multivariate Cox models adjusted for stratification factors and treatment group, MRD negativity was the strongest prognostic factor for progression-free survival (Table 2). Similar results were observed after having imputed values for missing MRD assessments (supplemental Table 6). As the summary of the results of the Cox models, adjusted probabilities of progression-free survival presented in Figure 2 and in supplemental Figures 4 and 5 show improvement in progression-free survival in those achieving MRD negativity irrespective of the treatment received (transplantation or RVD-only arms) or standard vs high-risk group or ISS stage I vs stage II or III.
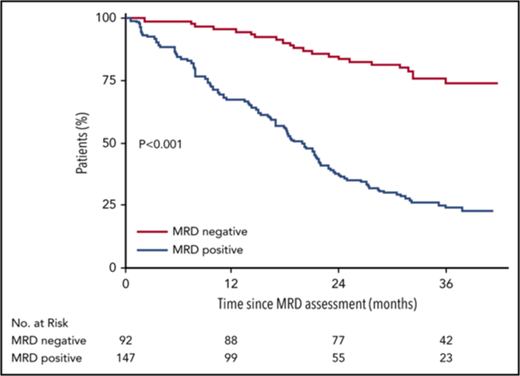
Kaplan-Meier survival curves for progression-free survival according to MRD status. (A) According to MRD status at the start of maintenance therapy. The median progression-free survival from the start of maintenance therapy was not reached among MRD− patients, and was 29 months among MRD+ patients. (B) After 12 months of maintenance therapy. The median progression-free survival from the completion of maintenance therapy was not reached among MRD− patients, and was 20 months among MRD+ patients. (C) Modified by the Simon-Makuch Method. The number of patients at risk in the MRD− group was 0 at the beginning of follow-up because of the time-dependent nature of MRD. Prior to the start of maintenance therapy, all patients were included in the MRD+ group; patients who were found to be MRD− on assessment during maintenance were switched to the MRD− group. The median progression-free survival from randomization was not reached among MRD− patients, and was 29 months among MRD+ patients.
Kaplan-Meier survival curves for progression-free survival according to MRD status. (A) According to MRD status at the start of maintenance therapy. The median progression-free survival from the start of maintenance therapy was not reached among MRD− patients, and was 29 months among MRD+ patients. (B) After 12 months of maintenance therapy. The median progression-free survival from the completion of maintenance therapy was not reached among MRD− patients, and was 20 months among MRD+ patients. (C) Modified by the Simon-Makuch Method. The number of patients at risk in the MRD− group was 0 at the beginning of follow-up because of the time-dependent nature of MRD. Prior to the start of maintenance therapy, all patients were included in the MRD+ group; patients who were found to be MRD− on assessment during maintenance were switched to the MRD− group. The median progression-free survival from randomization was not reached among MRD− patients, and was 29 months among MRD+ patients.
Probability of progression-free survival. (A) Adjusted for MRD status and treatment group, (B) cytogenetic risk status, and (C) ISS I and III grouped together, as hazard ratios in these subgroups were numerically identical (C). These curves were constructed according to the results of the Cox model performed on the 366 patients with known MRD status at start of maintenance therapy.
Probability of progression-free survival. (A) Adjusted for MRD status and treatment group, (B) cytogenetic risk status, and (C) ISS I and III grouped together, as hazard ratios in these subgroups were numerically identical (C). These curves were constructed according to the results of the Cox model performed on the 366 patients with known MRD status at start of maintenance therapy.
Overall survival
Nine deaths occurred among the 127 MRD− patients (7%): 4 in the RVD-alone group, and 5 in the transplantation group. Five of these deaths were myeloma related. Overall survival was significantly prolonged in MRD− patients vs MRD+ patients (Figure 3; supplemental Figure 7). Median overall survival was not reached in either group. Owing to the small number of deaths among patients who were MRD−, subgroup analyses were not performed.
Kaplan-Meier survival curves for overall survival according to MRD status. (A) Status at the start of maintenance therapy. The overall survival at 4 years after the start of maintenance therapy was 94% among MRD− patients, and 79% among MRD+ patients (adjusted hazard ratio for death, 0.24; 95% confidence interval, 0.11-0.54). (B) After 12 months of maintenance therapy. The overall survival at 3 years after the completion of maintenance therapy was 96% among MRD− patients, and 86% among MRD+ patients (adjusted hazard ratio for death, 0.26; 95% confidence interval, 0.10-0.68). (C) Modified by the Simon-Makuch method. The number of patients at risk in the MRD− group was 0 at the beginning of follow-up, because of the time-dependent nature of MRD. Prior to the start of maintenance therapy, all patients were included in the MRD+ group; patients who were found to be MRD− on assessment during maintenance were switched to the MRD− group. MRD was a strong prognostic factor for overall survival (adjusted hazard ratio, 0.16; 95% confidence interval, 0.08-0.32).
Kaplan-Meier survival curves for overall survival according to MRD status. (A) Status at the start of maintenance therapy. The overall survival at 4 years after the start of maintenance therapy was 94% among MRD− patients, and 79% among MRD+ patients (adjusted hazard ratio for death, 0.24; 95% confidence interval, 0.11-0.54). (B) After 12 months of maintenance therapy. The overall survival at 3 years after the completion of maintenance therapy was 96% among MRD− patients, and 86% among MRD+ patients (adjusted hazard ratio for death, 0.26; 95% confidence interval, 0.10-0.68). (C) Modified by the Simon-Makuch method. The number of patients at risk in the MRD− group was 0 at the beginning of follow-up, because of the time-dependent nature of MRD. Prior to the start of maintenance therapy, all patients were included in the MRD+ group; patients who were found to be MRD− on assessment during maintenance were switched to the MRD− group. MRD was a strong prognostic factor for overall survival (adjusted hazard ratio, 0.16; 95% confidence interval, 0.08-0.32).
Survival analyses, limited to the population of patients assessed by NGS and according to the change in MRD status before and after maintenance therapy, show a similar progression-free and overall survival for patients who maintained MRD negativity at both measurements or those who became MRD− after 12 months of maintenance. Their survival was significantly superior to those who were either MRD+ at both measurements or became positive at the later measurement (supplemental Figures 8-10).
Discussion
As a result of major improvements in clinical outcomes in recent years, the search for surrogate markers that enable early prediction of survival end points and reduce the time required to evaluate new treatments has become a major objective for the myeloma community. MRD is one of the most promising such biomarkers identified to date.19-21 In a recent meta-analysis of studies published between January 1990 and January 2016, MRD negativity was found to confer an ∼50% relative reduction in the risk of both progression and mortality.22 Although this risk reduction appears to be clinically important, a significant proportion of patients classified as MRD− still relapsed and died of disease. An explanation for this observation is the lower level of sensitivity of the older multiparametric flow cytometry techniques used in the studies included in the meta-analysis. With the development of more advanced technologies and the analysis of larger numbers of cells (10 million to 20 million), higher sensitivity is now observed.10
Using NGS,13 a technique that offers sensitivity at the 10−6 level, we are able to demonstrate that an ability to measure deeper response provides superior outcome; for example, MRD level below 10−6 is predictive of superior PFS compared with 10−5 or 10−4. With 10−6 sensitivity, we found MRD negativity to be a strong prognostic biomarker of progression-free survival and overall survival. This approach demonstrated a higher level of discrimination than had previously been achieved with the less sensitive multiparametric flow cytometry technique.14 However, recent data from the Spanish group showed that 10−6 sensitivity can be achieved by NGF as well. Thus, it is important to note that the methodology used to measure MRD is not as important, as long as we are able to measure deeper levels of MRD. For clinical practice and based on availability, any platform which achieves adequate sensitivity and reproducibility can be used. Our findings support and enhance the recently published International Myeloma Working Group criteria.23
Survival was prolonged in both high- and standard-risk myeloma patients who were MRD− vs standard-risk MRD+ patients. This finding raises the possibility that achieving MRD negativity may overcome certain adverse risk factors identified at diagnosis. These results, coupled with the lack of impact of disease stage or cytogenetic risk profile on MRD negativity rates, should be kept in mind when discussing prognoses with patients not only at diagnosis, but also during the different phases of treatment, as their prognosis could be substantially altered by their achieving MRD− status.
We found lower rates of MRD negativity among patients who received RVD alone than among those who underwent transplantation. However, due to the 80% relative risk reduction conferred by MRD negativity, regardless of treatment assignment, progression-free survival in MRD− patients in the RVD-alone group was similar to that in patients in the transplantation group with the same MRD status. Although the risk of progression was reduced in the transplantation group vs the RVD-alone group as a whole, it seems possible that the role of transplantation may be less clear in patients who achieve MRD negativity after induction therapy. As MRD assessments were only available at the start and end of maintenance therapy in our study, we were not able to test this hypothesis. Our study now provides the rationale to address these questions in future prospective trials to determine whether MRD status can be used to inform induction, consolidation, and/or maintenance treatments.
Despite higher sensitivity, biological relapses still occur in MRD− patients. This finding, along with the observed changes in MRD status over 1 year of maintenance therapy, highlight the requirement for serial MRD assessments to better assess the risk of progression. At present, the feasibility of monitoring MRD over time is limited by the invasive bone marrow biopsy procedures, but serum-based tests to facilitate this monitoring are currently undergoing clinical evaluation.
Although the characteristics of patients excluded from the analyses were comparable to those of patients who were included, we should emphasize that potential bias cannot be totally excluded because MRD data were unavailable for 50% of patients at the specified time points. The main reason that MRD was not assessed was the absence of a sample stored at the time of diagnosis to identify the clonal rearrangements.
In a significant number of patients, MRD evaluation was not feasible mainly because of lack of diagnostic sample for calibration. Because MRD evaluation by NGS was not planned in 2008 (because it did not exist), we did not make significant efforts to spare plasma cells for calibration. This point could be a limitation of NGS vs NGF, but, currently, we are able to calibrate >98% of the patients (H.A.-L., personal data). The main advantage of NGS vs NGF is the required number of cells to achieve the 10−6 threshold. With NGS, only 2 million cells are needed, vs at least 20 million for NGF. In this study, we used the Sequenta/Adaptive platform. Other platforms (commercial and academic) are available, but to our knowledge, the Adaptive MRD assay is the only one which is US Food and Drug Administration (FDA) approved.
In conclusion, NGS-determined MRD status enables the identification of patient subpopulations with highly different prognoses. Consequently, in the future, this end point could be used to inform treatment decisions and provide significant reassurance for myeloma patients who achieve MRD negativity, regardless of their cytogenetic risk profile or disease stage. It is also clear that MRD opens the door to evaluation of stratified therapy for multiple myeloma patients in future randomized clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Celgene and Janssen, National Institutes of Health, National Cancer Institute PO1 grants CA100707-12 and PO1-155258, and the Cancer Pharmacology of Toulouse-Oncopole and Region (CAPTOR) program. The Unité de Génomique du Myélome laboratory is sponsored by the Association Recherche Cancer.
Authorship
Contribution: A.P., V.L.-C., J.C., M.A., H.A.-L., and N. Munshi collected the data, performed analyses, and wrote the paper; V.C., M.M., T.W., and M.F. performed the MRD experiments; and all other authors provided samples and clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hervé Avet-Loiseau, Myeloma Genomics Laboratory, IUC-Oncopole, 1 Avenue Irene Joliot-Curie, 31059 Toulouse, France; e-mail: avetloiseau.herve@iuct-oncopole.fr; and Nikhil Munshi, Myeloma Department, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: nikhil_munshi@dfci.harvard.edu.