In this issue of Blood, Perrot et al reveal that patients with multiple myeloma (MM) who are eligible for transplantation and have undetectable minimal residual disease (MRD) by next-generation sequencing (NGS) during maintenance therapy have excellent outcomes.1
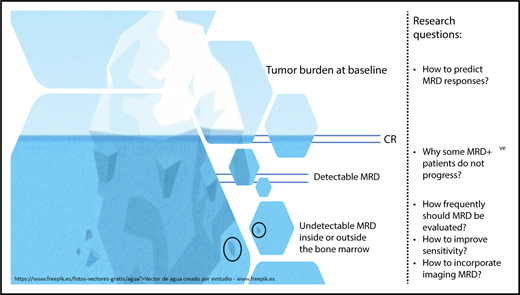
Tumor burden in MM before and after therapy is illustrated here by an iceberg. The achievement of complete remission (CR) is represented by the disappearance of measurable disease above the surface. Below the surface, MRD detected by sensitive methods is clinically relevant because it increases the probability of disease progression. Despite having a risk that is significantly lower, patients with undetectable MRD continue to be at risk of disease progression, which underlies the fact that chemotherapy-resistant MM cells persist either inside or outside the bone marrow. Thus, several research questions (enumerated in the rightmost portion of the figure) remain to be addressed.
Tumor burden in MM before and after therapy is illustrated here by an iceberg. The achievement of complete remission (CR) is represented by the disappearance of measurable disease above the surface. Below the surface, MRD detected by sensitive methods is clinically relevant because it increases the probability of disease progression. Despite having a risk that is significantly lower, patients with undetectable MRD continue to be at risk of disease progression, which underlies the fact that chemotherapy-resistant MM cells persist either inside or outside the bone marrow. Thus, several research questions (enumerated in the rightmost portion of the figure) remain to be addressed.
In 2016, the International Myeloma Working Group (IMWG) updated the response criteria and introduced definitions for MRD negativity based on NGS using the LymphoSIGHT platform (or validated equivalent method), flow cytometry using the EuroFlow standard operating procedure (or validated equivalent method), and positron emission tomography/computerized tomography (PET/CT).2 Promising results using NGS were reported by Martinez-Lopez et al in 2014,3 Korde et al in 2015,4 and Takamatsu et al in 2017.5 The results now reported by Perrot et al highlight the prognostic value of NGS-based MRD assessment in MM and establish it as the gold standard of molecular methods for evaluating treatment efficacy in this disease. NGS clearly supersedes the applicability of allele-specific oligonucleotide polymerase chain reaction (ASO-PCR; >90% vs 60% to 75%, respectively),3,4,6 and the progression rate of patients with undetectable MRD by NGS suggests a higher sensitivity of this method compared with ASO-PCR.6 Thus, the study by Perrot et al represents a milestone in NGS-based MRD, and it should stimulate complementary analyses to validate the other IMWG definitions for MRD negativity based on next-generation flow cytometry7 or PET/CT8 in large prospective series and assess whether these methods can match the ability of NGS to stratify risk in MM on the basis of patients’ MRD status.
In this important study by Perrot et al, patients treated according to the Intergroupe Francophone du Myélome (IFM) 2009 clinical trial9 who achieved undetectable MRD before maintenance therapy had an unprecedented reduction in the risk of progression and/or death of 78% after a median follow-up of 50 months. Furthermore, the benefit of achieving MRD negativity was independent of treatment group (lenalidomide, bortezomib, and dexamethasone [RVD] vs transplant), cytogenetic risk profile, or International Staging System disease stage at diagnosis. These data confirm previous findings based on multiparameter flow cytometry (MFC)10 and have 2 relevant consequences. (1) Because the benefit of undetectable MRD is independent of treatment, MRD could potentially be used as a clinical and surrogate end point. This suggestion is supported by the IFM 2009 results because the higher rates of MRD negativity by NGS or MFC after transplantation correlated with the superiority of this arm vs RVD alone.9 (2) Risk is dynamic. Patients with high-risk disease at diagnosis may enjoy progression-free survival (PFS) similar to that of standard-risk patients upon the achievement of MRD negativity. Importantly, the opposite is also true; patients with standard-risk disease but with persistent MRD have a median PFS only ∼12 months longer than that of patients with high-risk MM with persistent MRD.
In addition, patients who became MRD negative at the end of the 1-year maintenance regimen used in the IFM 2009 trial had outcomes similar to those who were already MRD negative before maintenance (supplemental Figure 8 in Perrot et al). These results suggest that achieving MRD responses with maintenance could be clinically relevant. At the same time, they also raise 2 important questions. (1) What would be the outcome of patients remaining or becoming MRD negative with more prolonged treatment? (2) How do we identify those patients who, despite remaining MRD positive, are benefiting from prolonged treatment because of surveillance of MRD clones?
Although a limitation of this study is the missing data (which the authors thoroughly addressed), perhaps the only sense of “slight disappointment” after its reading comes from the fact that, albeit at a much lower rate, patients with undetectable MRD continue to show a linear risk of relapse particularly after stopping treatment. Why have these patients progressed? Was the bone marrow sample inadequate? Was there extramedullary disease? How frequently should patients be monitored for MRD? How far does the limit of detection need to be pushed? How deep is the iceberg? (see figure). The MM-MRD community must continue working to answer these questions and build upon the seminal contribution made by Perrot et al in supporting the role of MRD as the most relevant clinical and surrogate end point.
Conflict-of-interest disclosure: J.F.S.-M. discloses participation in scientific advisory boards for Takeda, Janssen, Celgene, Novartis, Amgen, Bristol-Myers Squibb, Sanofi, Merck Sharp & Dohme, and Roche. B.P. discloses consulting fees from Celgene, Janssen, Merck, Novartis, and Takeda, funds for contracted research from Celgene, EngMab and Sanofi, and fees for non–continuing medical education/continuing education services from Amgen, Celgene, Janssen, and Takeda.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal