Abstract
Castleman disease (CD) describes a group of heterogeneous hematologic disorders with characteristic histopathological features. CD can present with unicentric or multicentric (MCD) regions of lymph node enlargement. Some cases of MCD are caused by human herpesvirus-8 (HHV-8), whereas others are HHV-8–negative/idiopathic (iMCD). Treatment of iMCD is challenging, and outcomes can be poor because no uniform treatment guidelines exist, few systematic studies have been conducted, and no agreed upon response criteria have been described. The purpose of this paper is to establish consensus, evidence-based treatment guidelines based on the severity of iMCD to improve outcomes. An international Working Group of 42 experts from 10 countries was convened by the Castleman Disease Collaborative Network to establish consensus guidelines for the management of iMCD based on published literature, review of treatment effectiveness for 344 cases, and expert opinion. The anti–interleukin-6 monoclonal antibody siltuximab (or tocilizumab, if siltuximab is not available) with or without corticosteroids is the preferred first-line therapy for iMCD. In the most severe cases, adjuvant combination chemotherapy is recommended. Additional agents are recommended, tailored by disease severity, as second- and third-line therapies for treatment failures. Response criteria were formulated to facilitate the evaluation of treatment failure or success. These guidelines should help treating physicians to stratify patients based on disease severity in order to select the best available therapeutic option. An international registry for patients with CD (ACCELERATE, #NCT02817997) was established in October 2016 to collect patient outcomes to increase the evidence base for selection of therapies in the future.
Introduction
Castleman described the first case of Castleman disease (CD) involving a single lymph node station, which is now referred to as unicentric CD.1 Characteristic histopathological features observed in CD lymph nodes include hyaline vascular, plasmacytic, and mixed variants.2,3 CD was later observed to affect multiple lymph node stations, which is known as multicentric Castleman disease (MCD).4 In 1995, human herpesvirus 8 (HHV-8) was found to be the etiologic agent of a plasmablastic variant of MCD occurring most commonly in HIV-infected or otherwise immunocompromised individuals.5-10 In HHV-8–associated MCD, viral interleukin-6 (IL-6), a homolog of human IL-6, promotes a proinflammatory state accounting for clinical symptomatology and laboratory abnormalities, such as anemia, hypoalbuminemia, and elevated C-reactive protein (CRP). In HHV-8–negative MCD, which comprises 33% to 58% of MCD cases, human IL-6 is the most common pathological driver, but the exact etiology is unknown; this entity is also referred to as “idiopathic MCD” (iMCD).11-15 We have proposed 4 etiological hypotheses, including autoimmune, autoinflammatory, neoplastic, and pathogenic mechanisms, which are now being actively investigated through the Castleman Disease Collaborative Network (CDCN).12,16
The presentation of iMCD is quite varied with some patients having mild constitutional symptoms, whereas others develop a life-threatening cytokine storm, organ failure, and death. The diverse clinical presentation calls for a treatment stratagem that takes into account the severity of the disease. Further complicating treatment recommendations is the existence of distinct iMCD subtypes. Some patients experience thrombocytopenia (T), anasarca (A), fever (F), reticulin fibrosis of the bone marrow (R), and organomegaly (O), but generally have normal γ-globulin levels, which has recently been referred to as the TAFRO subtype of iMCD.17,18 Other patients have more classic iMCD with features attributed to IL-6 excess, such as thrombocytosis and hypergammaglobulinemia, but less extreme anasarca.18,19 The TAFRO subtype often has more severe clinical symptomatology and worse outcome.18,20-22 We and others have reported that TAFRO patients have highly vascular lymph nodes and exhibit a different cytokine spectrum with elevated vascular endothelial growth factor (VEGF) levels, but milder elevation of IL-6.23-25
Four recent studies in HIV-negative HHV-8 status unknown (believed to be HHV-8–negative) MCD reported 5-year overall survival rates of 55% to 77%, reminiscent of the outcomes of malignant disorders, although a large series from tertiary specialty centers reported 1-year survival exceeding 90%.11,26-30 The poor outcome of iMCD may be due to several factors. First, there were no diagnostic criteria for iMCD prior to 2017, when the CDCN published the first-ever consensus diagnostic criteria for iMCD.23 Second, iMCD is a complex orphan disease with an incidence of 1000 to 1500 cases in the United States.31 Consequently, few physicians have substantive experience managing iMCD, and clinical trials are difficult to conduct. Third, there is a paucity of systematic studies to guide the treating physician, further compounded by the lack of uniform response criteria, hampering evaluation of treatment efficacy. Finally, there are no existing recommendations on how to use available treatment modalities in the context of disease severity.
iMCD has been treated with a wide variety of agents, including corticosteroids, rituximab, and chemotherapies. More recently, monoclonal antibodies (mAbs) targeting IL-6 directly (siltuximab) or the IL-6 receptor (tocilizumab) have been approved for iMCD therapy.32,33 However, a significant proportion of patients do not benefit from anti–IL-6 mAbs, and additional therapeutic options are needed for nonresponders, especially severely afflicted patients. Herein, we establish comprehensive guidance on the treatment of iMCD based on review of data from 344 patients, published literature, and expert opinion provided by a panel of physicians from the CDCN. The management of HHV-8–associated MCD and POEMS syndrome–associated MCD is well established and has been reported elsewhere.34-41
Methods
An international group of 42 participants from the United States, Japan, China, France, United Kingdom, Germany, Italy, Canada, Norway, and New Zealand, comprising experts in Hematology/Oncology, Hematopathology, Infectious Diseases, and Immunology, as well as 2 physician-patients with iMCD, embarked on the establishment of treatment guidelines for iMCD. The Working Group first met in December 2016 with a follow-up meeting in December 2017. Three additional Web-based teleconferences were held in August 2017, November 2017, and March 2018. All relevant English language publications from 1954 to 2017 were identified through PubMed and other databases using as MESH headings Castleman Disease, Multicentric, and TAFRO. All age groups, including pediatric cases, were included. Clinical trials conducted with siltuximab (#NCT00412321, #NCT01024036, and #NCT01400503) and tocilizumab were also reviewed. Five large data sets as well as individual case reports (see supplemental appendix 1, available on the Blood Web site) served as the primary evidence base.11,21,32,33,39
Based on the panel’s expert opinion, the impact of different therapeutic interventions was assessed in the context of disease severity, and recommendations for classification of severity and response criteria for evaluation of treatment were derived from the literature. The consensus focused on 3 main topics: (1) development of iMCD severity criteria, (2) treatment of iMCD, and (3) development of iMCD response criteria. Categories of evidence and consensus were modeled after those developed by the National Comprehensive Cancer Network (https://www.nccn.org/professionals/physician_gls/categories_of_consensus.aspx). A modified Delphi process comprising the integration of evidence provided by the literature and expert opinions was used to generate the final consensus statement contained in this paper, which was approved by all authors.
Data sharing statement
All data reviewed for the purposes of generating the consensus criteria were sourced from publicly available journal articles. A table describing the aggregate data as well as outcome calculations is available as a supplemental appendix.
Results
Management of iMCD
To serve as the evidence base for the development of iMCD management guidelines, a data set of iMCD clinical cases (n = 344) and treatment regimens (n = 479) was assembled (summarized in Table 1, complete data set in supplemental appendix 1).
Evaluation of iMCD severity
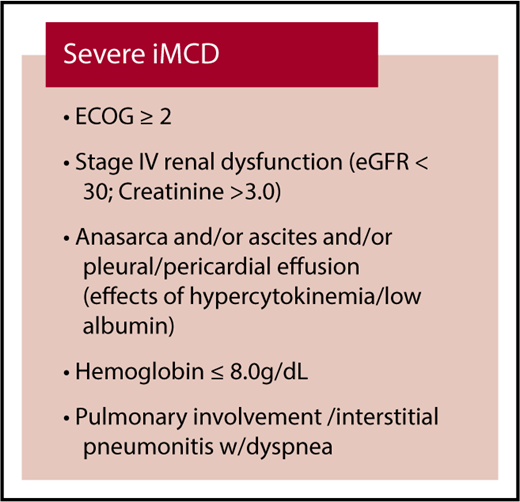
If a patient is suspected to have iMCD, a comprehensive set of testing is recommended to determine if the patient meets the consensus iMCD diagnostic criteria and assess disease severity (Table 2).23 Laboratory testing for inflammatory markers and organ dysfunction is indicated. Computed tomography (CT) should be performed to visualize the extent of the disease; CT–positron emission tomography (PET) scanning is a useful alternative, and high standardized uptake values (>6) should raise the suspicion of an alternative diagnosis (eg, lymphoma). The severity of iMCD spans a wide spectrum, with some patients exhibiting mild symptomatology, whereas others experience life-threatening organ failure. Based on expert opinion and review of the evidence base, we recommend assessing the severity of iMCD according to simple criteria (Figure 1) to inform the appropriate treatment choice as defined in Figure 2. These criteria are intended to segment patients according to their performance status and extent of organ dysfunction into 2 broad categories: nonsevere and severe. Patients with severe iMCD have evidence of organ dysfunction such as renal failure, anasarca, severe anemia, and pulmonary dysfunction resulting in poor performance status likely requiring critical care. Laboratory features include very high CRP levels (≥100 g/dL), marked hypoalbuminemia (≤2.0 g/dL), and thrombocytopenia (≤100 × 1012/L). Patients with lymphocytic interstitial pneumonitis can also progress to end-stage pulmonary fibrosis if inadequately treated.
CDCN severity classification for rapid assessment and allocation of therapy. Patients with severe iMCD must have at least 2 of the 5 criteria listed above. Patients should be classified as nonsevere iMCD if the above criteria are not met. ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate.
CDCN severity classification for rapid assessment and allocation of therapy. Patients with severe iMCD must have at least 2 of the 5 criteria listed above. Patients should be classified as nonsevere iMCD if the above criteria are not met. ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate.
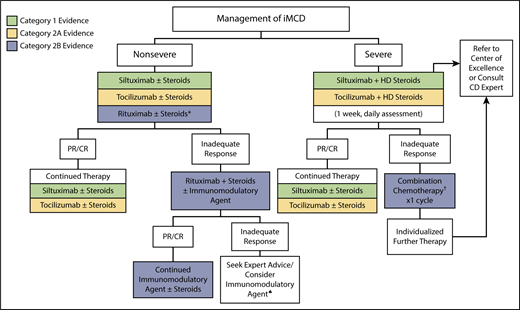
Treatment algorithm for iMCD. iMCD patients should be stratified for disease severity per Figure 1. For nonsevere iMCD, siltuximab is recommended as frontline therapy for patients with nonsevere iMCD. Tocilizumab can be used if siltuximab is not available or approved. Steroids are useful adjunctive therapy, and the dose can be tailored according to the severity of the disease. Patients responding to anti–IL-6 mAb therapy should be continued indefinitely. *For patients with mild symptomatology, a limited course of rituximab is an alternative option. Patients not responding to anti–IL-6 mAb therapy should be considered for rituximab-based therapy + steroids ± immunomodulatory/immunosuppressive agents. ♠Immunomodulatory/immunosuppressive agents for second- or third-line therapy include thalidomide, cyclosporine A, sirolimus, anakinra, or bortezomib, but we recommend consulting with an expert at this stage. For severe iMCD, severe disease must be closely monitored, as life-threatening events may occur in this population. Severely ill patients should be treated with siltuximab and high-dose steroids, but if no clear response occurs within 1 week (or if status worsens at any time), then combination chemotherapy should be considered. When possible, expert advice should be sought to identify the most appropriate therapy for a given patient. Further therapy is best individualized. †Examples of chemotherapy include R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), R-VDT-PACE (rituximab, bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, etoposide), or etoposide/ cyclophosphamide/rituximab. Siltuximab is the preferred anti–IL-6 therapy. However, in countries where siltuximab is not available or approved, tocilizumab can be used instead. Supporting evidence category 1, green boxes; category 2A, yellow boxes; category 2B, blue boxes. CTC, common toxicity criteria.
Treatment algorithm for iMCD. iMCD patients should be stratified for disease severity per Figure 1. For nonsevere iMCD, siltuximab is recommended as frontline therapy for patients with nonsevere iMCD. Tocilizumab can be used if siltuximab is not available or approved. Steroids are useful adjunctive therapy, and the dose can be tailored according to the severity of the disease. Patients responding to anti–IL-6 mAb therapy should be continued indefinitely. *For patients with mild symptomatology, a limited course of rituximab is an alternative option. Patients not responding to anti–IL-6 mAb therapy should be considered for rituximab-based therapy + steroids ± immunomodulatory/immunosuppressive agents. ♠Immunomodulatory/immunosuppressive agents for second- or third-line therapy include thalidomide, cyclosporine A, sirolimus, anakinra, or bortezomib, but we recommend consulting with an expert at this stage. For severe iMCD, severe disease must be closely monitored, as life-threatening events may occur in this population. Severely ill patients should be treated with siltuximab and high-dose steroids, but if no clear response occurs within 1 week (or if status worsens at any time), then combination chemotherapy should be considered. When possible, expert advice should be sought to identify the most appropriate therapy for a given patient. Further therapy is best individualized. †Examples of chemotherapy include R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), R-VDT-PACE (rituximab, bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, etoposide), or etoposide/ cyclophosphamide/rituximab. Siltuximab is the preferred anti–IL-6 therapy. However, in countries where siltuximab is not available or approved, tocilizumab can be used instead. Supporting evidence category 1, green boxes; category 2A, yellow boxes; category 2B, blue boxes. CTC, common toxicity criteria.
Nonsevere iMCD
iMCD patients who are not severely sick are typically diagnosed in the outpatient setting and have a good performance status without evidence of abnormal organ function, whereas other patients are more symptomatic and often exhibit an IL-6–driven inflammatory response that interferes significantly with their ability to function and work. Clinical symptoms may be intense enough to require hospitalization, albeit not in intensive care.
We recommend (category 1) using anti–IL-6 mAb therapy with siltuximab (11 mg/kg every 3 weeks) for all patients with nonsevere iMCD based on the high proportion of responders, the rigorous nature of the studies underlying the evidence base, and the low side-effect profile relative to other interventions. Siltuximab, which has been evaluated in a phase 1 trial (n = 34), a long-term safety study (n = 19), and a randomized, double-blind placebo-controlled phase 2 trial (n = 79), is presently approved in the United States, Canada, European Union, and Brazil, among other countries.33,42-47 In the phase 2 study, the only randomized controlled trial performed in iMCD to date, 79 patients were allocated to siltuximab 11 mg/kg every 3 weeks or placebo. Durable tumor and symptomatic responses were achieved in 18 of 53 patients in the siltuximab arm (34%; 1 complete response [CR], 17 partial responses [PRs]) vs 0 of 26 in the placebo arm. Nearly 60% of patients had a durable symptomatic response, and 31 patients continued to receive unblinded siltuximab.33 Although elevated pretreatment IL-6 levels are associated with a trend toward an increased likelihood of response to siltuximab, IL-6 levels should not be used to guide treatment decisions. In the phase 2 trial, there were iMCD patients with low/normal IL-6 levels who responded to siltuximab, whereas others with high IL-6 levels did not.45
If siltuximab is not available, tocilizumab (8 mg/kg every 2 weeks) may be used (category 2A). Tocilizumab, which has undergone an open-label, nonrandomized prospective study of 35 patients and been reported extensively in the literature, is approved for the treatment of iMCD in Japan. Like siltuximab, responding patients showed improvement in constitutional symptoms, normalization of abnormal laboratory markers such as CRP, hemoglobin, albumin, and immunoglobulin G, and reduction in lymphadenopathy with few significant adverse events.32,48,49 The most common side effects of both siltuximab and tocilizumab are mild thrombocytopenia, hypertriglyceridemia, hypercholesterolemia, and pruritus. The availability of siltuximab and tocilizumab varies among countries, and the choice between the 2 drugs is currently more dependent on indication within that country and access, as no head-to-head trials have been performed to compare efficacy.
If needed, first-line therapy with anti–IL-6 mAb should be accompanied by corticosteroid therapy for initial disease control. The existing data on corticosteroid monotherapy do not support its use due to limited long-term control and frequent relapses, except in countries where there is no access to mAb therapy.11,29,50-55 Combining data from published series, we noted a high treatment failure rate at 54% (Table 1). Nevertheless, corticosteroids can augment iMCD symptom control along with anti–IL-6 mAbs.32,33 Patients with more indolent disease can be treated with lower doses of adjunctive corticosteroids (eg, prednisone 1 mg/kg, or equivalent for 4-8 weeks followed by tapering; category 2B), whereas patients who are more symptomatic may require higher initial doses of corticosteroids (eg, methylprednisolone 2 mg/kg or equivalent) and more gradual tapering.
Careful inspection of the siltuximab and tocilizumab data and the clinical experience of the expert panel suggest that patients with a clear inflammatory syndrome as manifested by symptomatology and biochemical abnormalities are most likely to derive benefit from anti–IL-6 mAb therapy. In the tocilizumab studies, virtually all patients had increases in CRP, erythrocyte sedimentation rate (ESR), and fibrinogen as well significant anemia and hypoalbuminemia.32,48,49 Although no formal response criteria were employed, 86% of patients remained on therapy for at least 5 years.49 In contrast, the symptomatic response rates in patients treated with siltuximab were ∼60%, and the combined stringently defined end point of durable symptomatic and lymph node response was 34%. However, the patients in the siltuximab arm of the randomized trial were less severely affected as reflected by low scores on the MCD symptom scale as well as modest elevations in CRP and fibrinogen, and a median serum albumin that was in the normal range.33,45,47 Strict exclusion criteria for organ dysfunction and patient selection bias in the randomized siltuximab trial due to a placebo arm likely contributed to the milder phenotype in these patients. Of note, ad hoc analysis of the phase 2 data revealed that patients demonstrating more clinical and laboratory abnormalities included in the minor criteria of the iMCD diagnostic criteria had a greater response rate than those who did not.23
Responses in clinical symptomatology occur rapidly with anti–IL-6 mAb therapy and should be apparent after 3 to 4 doses.32,46 Laboratory indicators, including hemoglobin, CRP, ESR, and albumin, should mirror clinical improvements and be followed initially weekly and then biweekly until normalization.33,42,45 Of note, both siltuximab and tocilizumab give rise to spuriously elevated IL-6 levels for 18 to 24 months following the last dose. Therefore, serum IL-6 levels should not be used to assess response.14 Resolution of lymphadenopathy can be slow with anti–IL-6 mAb therapy with a median time to lymph node response of 5 months.33,46 This is because anti–IL-6 mAbs merely abrogate an important growth signal for lymphocytes and plasma cells, but do not have direct cytotoxic effects. Early response to therapy should be judged using the criteria provided in Figure 3, defining symptomatic and biochemical response rather than relying on reduction in lymph node size. Patients should be followed by serial CT scanning every 3 months until maximum response has occurred, after which the frequency of imaging can be reduced to 6 and later 12 months.
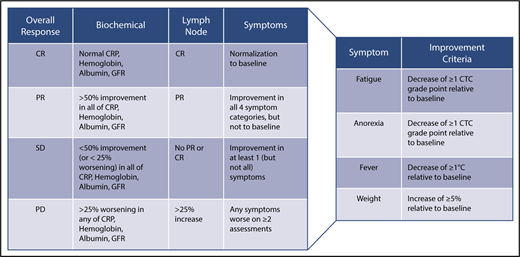
CDCN response criteria based on evaluation of biochemical, lymph node, and symptom response. Biochemical, lymph node, and response criteria have been detailed in the text. For lymph node response, Cheson criteria have been modified to include assessment of skin manifestations.42 An overall CR requires a complete biochemical, lymph node, and symptomatic response. An overall PR requires nothing less than a PR across all categories, but not meeting criteria for CR. Overall SD requires no PD in any of the categories and not meeting the criteria for CR or PR. An overall PD occurs when any category has a PD. Symptomatic and biochemical response evaluation should be done on a monthly basis until maximum response has been achieved. Radiological assessment of lymph node response by CT scanning is first recommended at 6 weeks and at 3-monthly intervals thereafter until maximum regression of lymph nodes has occurred. Lymph node response may take several months in patients treated with anti–IL-6 mAbs.
CDCN response criteria based on evaluation of biochemical, lymph node, and symptom response. Biochemical, lymph node, and response criteria have been detailed in the text. For lymph node response, Cheson criteria have been modified to include assessment of skin manifestations.42 An overall CR requires a complete biochemical, lymph node, and symptomatic response. An overall PR requires nothing less than a PR across all categories, but not meeting criteria for CR. Overall SD requires no PD in any of the categories and not meeting the criteria for CR or PR. An overall PD occurs when any category has a PD. Symptomatic and biochemical response evaluation should be done on a monthly basis until maximum response has been achieved. Radiological assessment of lymph node response by CT scanning is first recommended at 6 weeks and at 3-monthly intervals thereafter until maximum regression of lymph nodes has occurred. Lymph node response may take several months in patients treated with anti–IL-6 mAbs.
Clinical experience of the expert working group with siltuximab and data reported by Nishimoto et al for tocilizumab suggest that relapses occur on cessation of therapy.49 Indefinite continuation of anti–IL-6 mAb therapy in responding patients is therefore recommended. However, dosing intervals were safely extended to 6 weeks in 40% of iMCD patients in the long-term safety study of siltuximab, suggesting that dosing may be spaced out in some patients.44 If used in combination with other agents, steroids should be discontinued as early as possible to minimize side effects.
We recommend rituximab (375 mg/m2 × 4-8 doses) as a first-line alternative to anti–IL-6 mAb therapy for patients with nonsevere iMCD who do not have marked cytokine-driven symptomatology based on a more limited data set, because rituximab has not been subjected to systematic study in iMCD and data are confined to case reports or small series (category 2B evidence).56-61 Most papers report the use of rituximab along with conventional chemotherapies. Table 1 presents combined data on cytotoxic chemotherapy, which often includes rituximab as a component. In a recently published study of iMCD patients, the CR and PR rates with rituximab or rituximab-based chemotherapy regimens as first-line therapy were 20% and 48%, respectively. Rituximab-treated patients had inferior progression-free survival compared with those managed with siltuximab.21 In 2 further series, approximately half of the iMCD patients failed rituximab.11,39 Despite the lack of rigorous evaluation, the available data and expert opinion do support a role for rituximab monotherapy in the treatment of nonsevere iMCD patients for whom it would be reasonable to give a limited course of therapy rather than indefinite anti–IL-6 mAb treatment.
It is important to note that ∼50% of iMCD patients will not achieve a satisfactory response to first-line anti–IL-6 therapy. Failure to achieve a satisfactory response, defined as PR or CR (Figure 3), to first-line therapy should prompt reevaluation of the original diagnosis to rule out an alternative diagnosis, such as lymphoma. Anti–IL-6 mAb treatment does not need to be continued if it was not effective in first-line therapy. Second-line therapy should comprise rituximab to which immunomodulatory/immunosuppressive agents (Figure 2), and steroids may be added. Thalidomide has been combined with rituximab and steroids because it downregulates IL-6 expression and has antiangiogenic properties by modulating VEGF. Thalidomide has induced remissions in iMCD as a single agent and has also been valuable in combination with rituximab in both HHV-8–associated MCD and iMCD (Stephen Schey, Guys and St. Thomas' NHS Foundation Trust, oral communication, August 2017).62-64
Third-line therapy for patients who fail both anti–IL-6 mAbs and rituximab is less well defined. Cytotoxic chemotherapies have a high response rate in our pooled data analysis (78%), but treatment failure with relapses are common (42%) and toxicities are significant (Table 1). Therefore, the consensus opinion is to avoid cytotoxic chemotherapy unless the patient progresses to severe iMCD. We recommend use of an immunomodulatory/immunosuppressive agent because these agents have less toxicity than chemotherapy and have similar efficacy (69% response), albeit in fewer case reports.62-72 These agents include cyclosporine A, sirolimus, thalidomide, lenalidomide, bortezomib, the IL-1β receptor antagonist anakinra, retinoic acid derivatives, and interferon-α.62,63,65-73 Cyclosporine A has been used most extensively in iMCD-TAFRO cases, particularly to improve persistent ascites and thrombocytopenia.20,74-77 Anakinra, which blocks the IL-1β receptor and presumably NF-kB signaling, has been reported as successful treatment of a siltuximab-refractory iMCD patient.67,68
Severe iMCD: how to treat the critically ill patient
Based on published data, the proportion of patients with severe iMCD, who have marked organ dysfunction, poor performance status, and require critical care, is estimated to be 10% to 20%.11,21 These patients should be promptly started on a high-dose steroid regimen (eg, methylprednisolone 500 mg daily) together with siltuximab. For pharmacokinetic reasons, an accelerated, weekly dosing schedule of siltuximab may be used for 1 month. Patients who immediately respond should continue on siltuximab at every 3-week intervals indefinitely and slowly taper steroids.
There is consensus in the Working Group that patients with severe iMCD are at significant risk of mortality, and expert advice should be sought. Severe iMCD may not respond immediately to high-dose steroids and anti–IL-6 mAbs, which can take weeks to achieve steady state concentration. Still others may never respond to anti–IL-6 mAbs. Therefore, aggressive intervention with multiagent chemotherapy should be considered as early as necessary (any sign of deterioration or after 1 week of no response to siltuximab, whichever comes first) to ablate the hyperactivated immune system and stem the cytokine storm. Chemotherapy regimens, including those for lymphoma: R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), or CVP (cyclophosphamide, vincristine, prednisone); myeloma: VDT-ACE-R (bortezomib, dexamethasone, thalidomide, doxorubicin, cyclophosphamide, etoposide, rituximab); or etoposide/cyclophosphamide–containing regimens as used for hemophagocytic lymphohistiocytosis have all been employed.18,50,54,78 Combination chemotherapy is appropriate in poor performance status patients, including those requiring treatment in the intensive care unit, as control of the cytokine storm can be life-saving and bring about rapid improvement. As per Table 1, cytotoxic chemotherapy has the highest overall response rate (78%), but considerable toxicities and frequent relapses deter its use outside of the most severe setting when the risk/benefit analysis is skewed.29,52,79
The subsequent management of severe iMCD patients who fail to respond to anti–IL-6 mAb or the first cytotoxic chemotherapy regimen, or those who relapse, is not well defined and is mostly done on an ad hoc basis taking into account any previous response, clinical status, comorbidities, and cytokine profile. Patients who have elevation of IL-6 prior to starting anti–IL-6 mAb therapy may still benefit from extended therapy with anti–IL-6 mAb, even if they did not respond during the acute episode, whereas others may respond to immunomodulators/immunosuppressants or salvage cytotoxic therapy more commonly used in plasma cell malignancies (eg, VTD [bortezomib, thalidomide, dexamethasone]). Autologous and allogeneic stem cell transplantation has only been reported in a few cases with mixed results and are therapies of last resort.80-83
Severe iMCD often presents as the TAFRO subtype. Our analysis of 49 published iMCD-TAFRO cases with treatment data revealed that corticosteroids, anti–IL-6 mAbs, cytotoxic chemotherapies, and cyclosporine A are most often used. These agents demonstrate initial similar efficacy to the other cohorts, but higher rates of treatment failures and relapses (Table 3). Based on the available evidence, we recommend following the same treatment algorithm as for other cases of iMCD that is dependent on disease severity and initiate therapy with anti–IL-6 mAb therapy with or without corticosteroids. Among TAFRO cases, cyclosporine A can be useful therapy for anti–IL-6-refractory cases particularly to improve persistent ascites and thrombocytopenia.21,74-77 The Japanese TAFRO research group recommends high-dose steroids, tocilizumab, and cyclosporine A for patients with TAFRO syndrome.84 A comprehensive analysis of a treatment-refractory iMCD-TAFRO patient who sustained multiple relapses after repeated cycles of chemotherapy showed upregulation of the mTOR pathway, and remission was successfully maintained with sirolimus.85 Early data suggest that the proteomic profiles of classical and TAFRO-iMCD are different, supporting the notion that there may be diverse chemokines/cytokines driving the symptomatology across the iMCD spectrum.24,25
Evaluation of response
As is evident from the review of published literature, criteria for response to treatment of iMCD have thus far not been agreed upon. In the tocilizumab study, the primary end point was based on improvements in specific laboratory tests, but there was no aggregated response definition.32 The phase 1 siltuximab study used Cheson criteria for lymph node response modified to assess the skin lesions of iMCD. This trial introduced a clinical benefit response assessing 6 iMCD-related clinical features.42,86 In the phase 2 registration study of siltuximab, lymphadenopathy was similarly assessed, but the symptomatic response was evaluated by the investigators using a complex 34 iMCD-related symptom score.33
The Food and Drug Administration, in its approval of siltuximab, commented on the necessity of a composite response assessment for iMCD.87 Therefore, our expert panel established a composite end point for evaluating response taking into account all cardinal features of the disease: (a) objective biochemical markers of inflammatory response and organ function (hemoglobin, CRP, albumin, estimated glomerular filtration rate); (b) lymph node size; and (c) clinical symptoms (fatigue, anorexia, fever, weight change) as assessed by the clinician (Figure 3).
A biochemical CR requires normalization of all values compared with baseline. In a PR, there is 50% to 99% improvement in all laboratory values. In patients with SD, there is a <50% improvement in all laboratory values or <25% worsening in any laboratory indicators. Progressive disease (PD) indicates a >25% worsening in any of the laboratory markers. Lymph node response is assessed using modified Cheson criteria as previously published.42,86 Last, 4 important clinical symptoms are assessed using the National Cancer Institute Common Terminology Criteria of Adverse Events (version 4). A symptomatic CR requires normalization of all symptoms. PR requires improvement in the grades of all 4 symptoms, but they do not have to return to baseline. SD requires not meeting the criteria for PR or PD, which occurs if any symptoms worsen on ≥2 assessments 4 weeks apart. Evaluation of overall response requires integration of the 3 response categories as defined in Figure 3.
Discussion
The published diagnostic criteria of iMCD, together with the recognition of the TAFRO-iMCD subtype, provide a framework for recognizing different clinical entities on the CD spectrum.18,23 We present the first formal guidelines for the treatment of iMCD, depending on symptom severity. Based on the response criteria used in the literature and our clinical expertise, we propose composite response criteria addressing all relevant features of the disease to evaluate treatment in both clinical practice and future studies. The present guidelines should assist physicians with selecting therapy and evaluating response, thereby improving patient outcomes. The preferred treatment of nonsevere iMCD is siltuximab, whereas for some patients with limited symptomatology, a short course of rituximab is an alternative option. Patients with severe iMCD are a challenge and may require early intervention with combination chemotherapy to avoid a fatal outcome. Not all patients will benefit from siltuximab therapy, especially those who have a very mild inflammatory syndrome, or on the other end of the spectrum, severely ill patients who require a rapid response. Last, it has become clear that iMCD has a pleomorphic cytokine profile and that the disease is not driven by IL-6 in all.
There are several important limitations to our treatment recommendations. It is important to highlight that most recommendations were reached by consensus and are not supported by prospective, randomized data. Because of the rarity of the disease, there are no clinical studies available comparing treatment modalities such as chemotherapy, rituximab, and anti–IL-6 mAbs. Although the evidence base included clinical trial data and the largest collection of treatment data analyzed to date, it should be noted that case reports and retrospective case series with short follow-up durations make up a large portion of cases, which are subject to publication bias of successful uses of novel agents. In addition, the various studies used different criteria for assessing response (CR, PR, “response”) (eg, the threshold for a CR in a randomized controlled trial is likely different from that in a case report). Therefore, we aggregated all response categories into 1 global response category, which is listed in Table 1. We included a broad range of data from multiple sources to minimize these limitations. Although anti–IL-6 mAbs are an important contribution to the therapeutic armamentarium for iMCD, treatment must be continued long term. The CDCN established an international registry (www.CDCN.org/ACCELERATE), which collects data pertaining to treatment and outcome, to increase the evidence base for selection of therapies in the future. Ongoing research will focus on defining the etiology and pathogenesis of this complex and rare disease to promote the development of better and more targeted therapies, particularly for patients who do not benefit from anti–IL-6 mAb administration.
The online version of this article contains a data supplement.
Acknowledgments
The CDCN coordinated the meetings during which the treatment guidelines were developed. The authors of the guidelines had full responsibility for the consensus: building process/methods, data interpretations, treatment recommendations, and writing of the report.
Authorship
Contribution: All authors were responsible for the conceptualization of this manuscript and participated in the generation of these consensus treatment guidelines; F.v.R. and D.C.F. wrote the paper; and K.S. and A. Greenway edited the paper and made figures and tables.
Conflict-of-interest disclosure: F.v.R. has received research funding from Bristol Myers-Squibb and Janssen Pharmaceuticals and has served on advisory boards from Janssen Pharmaceuticals. D.C.F. has received research funding from Janssen Pharmaceuticals. R.W. and C.C. have received research funding from Janssen Pharmaceuticals and served on advisory boards for Janssen Pharmaceuticals. P.V. has served on advisory boards for Janssen Pharmaceuticals. S.F. has received consultancy fees and speaker honoraria and served on advisory boards for Janssen Pharmaceuticals. A.F. has received honoraria from Janssen Pharmaceuticals. T.S.U. has received research funding Genentech, Merck, and Celgene via Cooperative Research and Development Agreements with the National Cancer Institute and has a patent for an immunomodulatory compound for KSHV malignancies (Inst). R.K. has received research funding from Incyte, Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health, and Konica Minolta, consultant fees from LOXO, Actuate Therapeutics, Genentech, and NeoMed, as well as speaker fees from Roche, and has an ownership interest in Curematch, Inc. D. Simpson has received honoraria and research funding from Janssen Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Frits van Rhee, Myeloma Center, University of Arkansas for Medical Sciences, 4301 West Markham, Mail slot 816, Little Rock, AR 72205; e-mail: vanrheefrits@uams.edu.