Plasma hyperviscosity is a rare complication of both monoclonal and polyclonal disorders associated with elevation of immunoglobulins. Asymptomatic patients with an elevation in the serum viscosity do not require plasma exchange, and the majority will have other indications for therapeutic intervention. For patients with hemorrhagic or central nervous system manifestations, plasma exchange is the therapy of choice and is relatively safe. Viscosity measurements are not required to initiate therapy if the index of suspicion is high and the clinical presentation is typical. However, patients should have a sample sent for confirmation of the diagnosis. Whole-blood hyperviscosity is seen in patients with extreme elevation of the red cell and white cell count. Phlebotomy of patients with primary and secondary elevation of the red cell count is a well-established therapy.
Patient summary
A 58-year-old man was diagnosed with immunoglobulin A (IgA) κ multiple myeloma. Over a period of 8 years, he was treated with multiple chemotherapeutic regimens with response and subsequent relapse. At 66 years of age, he presented with epistaxis, gait instability, and somnolence. The epistaxis was both nostrils and could not be stopped with pressure or cautery. His hemoglobin was 7.9 g/dL, and platelet count was 34 × 109/L. Total protein was 12.3 g/dL, M spike was 6.6 g/dL, and quantitative immunoglobulin was 7220 mg/dL. A serum viscosity was drawn and sent to the laboratory while plasma exchange was initiated; 3479 mL of plasma was removed and replaced with 3304 mL of normal serum albumin over 68 minutes. The serum viscosity came back at 13.6 centipoise (cP). After the first plasma exchange, the viscosity was reduced to 3.6 cP. A second plasma exchange was performed the following day; 3535 mL of plasma were removed and were replaced with 3302 mL of albumin over 70 minutes. The viscosity was then measured as 1.8 cP. Although epistaxis resolved immediately, the patient’s mentation did not clear and he remained somnolent. Salvage chemotherapy was initiated but failed, and the patient died 17 days later of refractory multiple myeloma.
Introduction
Viscosity of a fluid is a measure of its resistance to flow based on shear stress. This corresponds to the concept of thickness. The best example would be automotive engine oil, which is available in multiple viscosities defined by the Society of Automotive Engineers (SAE) followed by a number. Intuitively, it is clear that it is easier for flow to occur through a garden hose rather than a drinking straw. Flow is also easier to achieve in a shorter tube than a longer tube. Flow increases at higher temperatures and at higher driving pressures (systolic blood pressure). Viscosity is the result of friction between molecules moving through a tube. Fluids in the center of the tube actually move at a higher velocity than fluids that abut the edge of the tube (endothelium) where drag occurs. Flow actually occurs as multiple concentric cylinders from the periphery to the center; flow is most rapid in the center.
The physics of viscosity
Isaac Newton hypothesized that viscosity is independent of flow conditions and is an intrinsic property of a fluid. Fluids such as water, plasma, and serum fulfill this Newtonian hypothesis. As a result, viscosity remains the same over shear rates that vary up to 3 logs.1 All Newtonian fluids can have their viscosity described by a single numeric value.
However, whole blood is non-Newtonian as the particles (cells) suspended in the plasma move away from the periphery of the blood vessel and stream through the center of the axis of flow. In fact, the cells glide on a thin layer of plasma along the vessel wall. Non-Newtonian fluids, such as whole blood, have viscosity that is dependent on ambient conditions that must be specified in order to determine the viscosity of the whole blood. The viscosity of whole blood varies based on temperature, driving pressure, shear, and vessel radius.
The mathematics of viscosity were first described by Poiseuille in 1828,2 when he determined that the volume of liquid flowing in a tube of small diameter was proportional to the bore of the tube and the pressure difference at both ends when temperature is fixed.3 Fahey coined the clinical term “hyperviscosity syndrome” (HVS) in 1965.4 Between 1932 and 1937, reports of increased serum viscosity in multiple myeloma5 followed by the description by Waldenström in 1944 of an elevated viscosity in patients with macroglobulinemia were noted.6 This included the triad of mucosal bleeding, visual alterations, and neurological dysfunction. The first report of plasma exchange occurred in 1959.
The physics of viscosity would suggest that the smaller the vessel, the greater the viscosity, and the greater the risk of shear damage to the vessel. This would predict that hemorrhage would occur at the capillary level, which is not what is seen clinically. The highest viscosity is in the postvenule system and was described by Fahraeus in 1929.7 Viscosity is actually lower in the capillary due to central streaming of deformable red blood cells.
Viscosity can be measured in 1 of 2 ways: (1) as an absolute value where the physical unit is the centipoise [cP = 1N/(M2sec)]. The viscosity of water is 0.894 cP. The viscosity of serum is ≤1.5 cP, SAE10 motor oil is 65, olive oil is 81, and SAE40 motor oil is 319 at 25°C. (2) In relative value units, the viscosity of serum and plasma are not measured directly but are usually expressed as a value relative to water, which is set at 1.0 (this has no units). The relative viscosity of serum in this measurement system is 1.7.
The measurement of viscosity is done in a viscosimeter. Historically, this was done using the Ostwald viscosimeter. Its use dates back to the turn of the 20th century and involves measuring the flow of serum or plasma through a capillary tube connected via a U-tube to 2 reservoirs.8 This requires significant technical expertise to be reproducible. It involves the use of a stopwatch to measure the flow in seconds, first of water and then remeasuring serum or plasma. The ratio between the 2 flow times determines the relative viscosity of the fluid.9 Today, in commercial laboratories, mechanical direct measurement is preferred because the Oswald tube requires 5 mL of serum and requires experienced technicians for it to be reproducible. Mayo Medical Laboratories uses the Sonoclot Coagulation Analyzer, which can do coagulation testing, thromboelastography,10 as well as viscosity measurement. This requires between 0.65 and 1.5 mL of serum and operates on the principle of the power required to oscillate a probe at constant rate.11 The increased power required in sera with increased viscosity is calibrated by standards of known viscosity and can be completed in 15 minutes. Because measurement of viscosity by this technique requires the construction of a standard curve, the test is usually reserved for central laboratories where the volume of samples justifies the production of a control curve. Serum and plasma viscosity management, however, should always be based on a direct measurement of serum viscosity even if intervention is required before the results subsequently become available, as was done in the patient presented.12
In serum and plasma, proteins determine the viscosity level. The 3-dimensional structure of the protein is important. Spherical proteins rotate through the plasma and contribute very little. Large linear proteins spin end over end and raise viscosity disproportionately. This is true of both fibrinogen13 in plasma and immunoglobulins in serum. IgM, with its molecular weight of 950 kDa, has a high axial length-to-width ratio and, therefore, has high intrinsic viscosity and raises the plasma viscosity at levels far below those of plasma proteins such as IgG and IgA. The median IgM level producing hyperviscosity in Waldenström macroglobulinemia (WM), reported from the University of California San Francisco (UCSF), is in the range of 5000 mg/dL. Hyperviscosity with IgG myeloma circulating as a monomer with a molecular weight of ∼180 kDa often requires a level in excess of 10 000 mg/dL.14 IgA is intermediate as it circulates as a dimer, and clinically important hyperviscosity can be seen with levels as low as 7000 mg/dL, as seen in the patient example.15
Clinical syndromes associated with serum hyperviscosity
Types 1 and 2 cryoglobulinemia (type 2 cryoglobulins are also rheumatoid factors present in high titer) are well-described causes of hyperviscosity as the gelling phenomenon that occurs in the peripheral circulation rapidly raises the viscosity of serum and is highly temperature dependent.16 Polyclonal elevations of immunoglobulins can also produce hyperviscosity.17 Sjögren syndrome, which is strongly associated with marked polyclonal hyperglobulinemia, has been reported to cause HVS, responsive to plasma exchange.18
Uncontrolled HIV infection with liver disease can produce extreme polyclonal hyperglobulinemia and hyperviscosity. High titers of rheumatoid factor, where a polyclonal IgM and polyclonal IgG form a high molecular weight complex, can produce hyperviscosity19 as can IgG4 disease. Rare instances of IV immunoglobulin infusion, particularly in the neonatal setting, have been reported to produce transient hyperviscosity requiring therapeutic intervention.20,21 Causes of hyperviscosity are listed in Table 1.
Whole-blood hyperviscosity
Whole-blood viscosity is generally not measured in clinical laboratories, and management is based primarily on guidelines of target hematocrit (goal hematocrit <45%) or levels of blast count (<100 000/μL) to justify intervention. The viscosity of whole blood increases as the red cell count and hematocrit increase.22 Whole-blood viscosity is ∼4.5 cP at a shear rate of 200 per second. At high shear rates, red cells disperse, form ellipsoids, and flow becomes streamlined. However, as the hematocrit exceeds 50%, there is a rapid increase in viscosity. The viscosity increases threefold between a hematocrit of 50% and 80%.23 If red cells were not deformable, blood would gel at a hematocrit above 70; but because red cells are highly deformable, blood continues to flow at high hematocrit levels. However, when red cells become rigid and nondeformable, such as in sickle cell anemia, viscosity of blood rises rapidly at low hematocrits and causes arterial hypertension.24
White blood cells contribute to the viscosity of whole blood. White cells are rigid and nondeformable due to their nuclei and cytoplasmic granules. They contribute, per cell, much more to the whole-blood viscosity; but because the normal red cell count is 1000 times that of the white cell count, the contribution is minimal until marked leukocytosis occurs. Whole-blood viscosity, therefore, depends on hematocrit, the reversible aggregation of red cells, the mechanical properties of red cells, as well as the viscosity of plasma, which is affected by fibrinogen, albumin, and globulin levels.25
Clinical manifestations associated with hyperviscosity
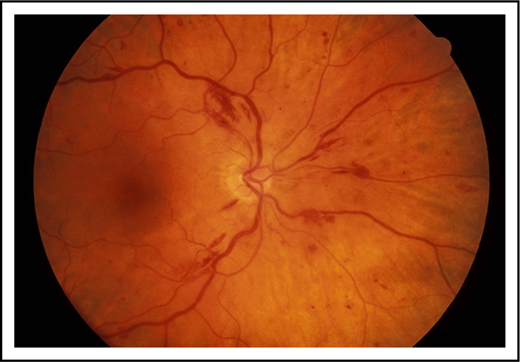
The classic triad of hyperviscosity includes mucosal bleeding, visual abnormalities, and neurological abnormalities. The most reliable clinical manifestations of serum hyperviscosity are oronasal bleeding and visual disturbances (Table 2). Whenever HVS is suspected, ophthalmoscopy should be performed. Viscosity is highest in small venules, and the drag that is exerted by viscous fluids will effectively tear the venule wall if there is not adequate underlying tissue support. Therefore, bleeding is seen where the veins are exposed on the surface with no superimposed epithelial tissue. This most commonly occurs in the lining of the nose, the gum, the retina, the lumen of the gastrointestinal tract, and the surface of the brain. A common scenario is a patient seeking evaluation in an emergency room because of epistaxis. Typically, this is intractable and bilateral. Patients frequently undergo cautery and are dismissed only to return, often on multiple occasions, at which point a complete blood count is obtained.
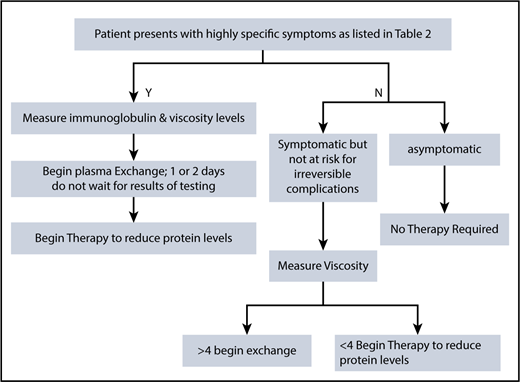
In patients with multiple myeloma and WM who have sufficient tumor burden to produce an immunoglobulin protein >5000 mg/dL, anemia is invariably found and triggers a more extensive evaluation, leading to the diagnosis. Patients with hyperviscosity tend to have plasma volume expansion; hence, the actual anemia may be partially dilutional and not well reflected by standard measurement. Although there is no scientific proof, there is a theoretical argument that could be made to withhold transfusion in hemodynamically stable patients until serum viscosity is normalized in order to avoid the rheological impact of adding red cells to the circulation. The presence of epistaxis or gingival bleeding requires a retinal examination because hemorrhages can occur without visual symptomatology (Figure 1). The presence of retinal changes consistent with hyperviscosity and not due to coexisting conditions, such as hypertension or diabetes, demands therapeutic intervention. Somnolence, diplopia, and dizziness are much softer signs because, in these patients, these symptoms may reflect plasma volume expansion, anemia, and electrolyte disturbances that often accompany newly diagnosed Waldenström and multiple myeloma. Plasma exchange should not be delayed awaiting viscosity results, but a sample should be sent for confirmation of the clinical impression. The sample can be drawn and sent while plasma exchange is initiated. Subsequently, the procedure can be halted if the diagnosis is not laboratory validated. It is uncommon for hyperviscosity to occur with levels of the monoclonal immunoglobulin below 4000 mg/dL, and it is our policy not to measure the viscosity level below these immunoglobulin levels unless there are compelling symptoms or physical findings to justify this measurement.
Retinal photograph. Patient with hyperviscosity demonstrating retinal hemorrhages and venous tortuosity.
Retinal photograph. Patient with hyperviscosity demonstrating retinal hemorrhages and venous tortuosity.
The most serious ophthalmic manifestation of hyperviscosity is bilateral central retinal vein occlusion.26 This has been reported in all forms of hyperviscosity, including IgG multiple myeloma.27
Headache, nonspecific light-headedness, and hearing loss have been reported as symptoms of hyperviscosity but lack the same specificity of epistaxis and gingival and retinal hemorrhage. Of 182 patients with WM, vestibular symptoms were noted in 9 and hearing loss in 7. Hearing loss from hyperviscosity has been attributed to stagnant blood in cochlear veins. Pulmonary hypertension has been described with dyspnea secondary to hyperviscosity.
On routine screening in a series from the Czech Republic, increased viscosity was found in 20.5% of 1400 sera, with HVS in 44 patients (3%). In Waldenström, the mean monoclonal protein concentration was 4.1 g/dL. In IgG myeloma with hyperviscosity, the mean monoclonal protein count concentration was 6.4 g/dL.28
Studies have been done looking at retinal vessel diameter, and there is a correlation between the diameter and increasing serum IgM levels. The mean IgM level of patients with the earliest retinal changes was 5442 mg/dL.29 It appears that each patient with Waldenström has a distinct viscosity threshold for the development of symptoms. Therefore, one cannot specify a specific viscosity level above which patients should receive prophylactic plasma exchange in the absence of symptoms. Hyperviscosity is rarely the sole indication for therapeutic intervention. Most patients who have monoclonal protein concentrations high enough to produce hyperviscosity generally have significant other disease-defining features that require therapy, such as anemia or adenopathy in Waldenström disease, and bone lesions or renal insufficiency in multiple myeloma. Although the International Myeloma Working Group does not specifically identify HVS as a myeloma-defining event, clearly its presence demands chemotherapeutic intervention. In a report of 20 episodes of hyperviscosity in 14 patients, 5 had multiple myeloma, 4 had Waldenström, 4 had Sjögren syndrome,30 and 1 patient had non-Hodgkin lymphoma. Plasma cell dyscrasia caused 70% of the episodes. The median number of plasma-exchange sessions required to produce control of hyperviscosity symptoms was 2.31 Among 825 newly diagnosed Waldenström patients reported from the Dana-Farber Cancer Institute, 14% developed symptoms consistent with hyperviscosity, but only a minority of patients had the viscosity level measured. It is unclear whether the symptoms were truly direct manifestations of elevated serum viscosity. In this cohort, a serum IgM level of >6000 mg/dL was associated with a median time to clinically identified hyperviscosity of 3 months.32 Hyperviscosity does not impact overall survival. In a recent report of 687 Mayo Clinic patients, HVS was observed in 13.5%. After excluding the patients who developed HVS at diagnosis, analysis showed that IgM > 4600 mg/dL (hazard ratio, 3.1 [1.3-8.9]; P < .0013) and viscosity > 2.2 cP (hazard ratio, 7.6 [3.2-20.5]; P < .0001) were independent predictors of development of hyperviscosity. This group had a 36% cumulative probability of developing hyperviscosity at 6 months. Twenty-four patients (22%) with serum IgM > 6000 mg/dL at diagnosis were otherwise asymptomatic (smoldering WM), not requiring therapy at diagnosis. The median viscosity for this cohort was 1.6 cP (1.0-4.0 cP) and no patients had viscosity >4 cP. Of these 24 patients, none developed HVS, subsequently, and median time to frontline treatment was 9 years. This points out the need to carefully assess for symptoms and not to initiate therapy based on numbers alone (Figure 2). The majority of patients with a baseline IgM of 6000 already have an indication for the initiation of therapy based on 2003 consensus guidelines. However, high serum IgM levels alone do not merit chemotherapy if the patient is otherwise asymptomatic. It is well recognized that rituximab can cause a flare of the IgM protein. When the levels are below 4000, even a flare of 30% is unlikely to produce symptomatic hyperviscosity. It is unproven that preemptive plasma exchange in asymptomatic patients with IgM levels >5000 prevent complications when compared with close monitoring following rituximab and the initiation of treatment if symptoms develop. However, many would not agree with this and use preemptive plasma exchange prior to rituximab-based therapy when IgM is very elevated.
Transient increases in the IgM level can occur after rituximab-based therapy for newly diagnosed patients.33 These flares can occur in 22% of patients and can produce symptomatic hyperviscosity after the initiation of therapy.34 Recently, protocols have been developed wherein rituximab is not initiated until after cycle 1 of a multiagent regimen to allow for reduction of the IgM level so that symptomatic flare does not occur when rituximab is introduced.
Treatment of HVS
The standard of care for managing hyperviscosity of the plasma is therapeutic apheresis, and guidelines on the use of apheresis have been previously published, and hyperviscosity is considered category A evidence for plasma exchange.35 There are 2 methods of plasma exchange. The first, used predominantly in many European countries, is centrifugation where a rapidly rotating bowl separates whole blood into the cellular component and the plasma compartment. The plasma is decanted and discarded. Replacement fluid, which can be saline but more typically is 5% serum albumin, is reinfused. The second technique involves double-membrane filtration. In double filtration, 2 hollow fiber filters with different pore sizes are required.36 The primary filter is the same that is used for plasma exchange using centrifugation as a plasma separator. Filtered plasma then flows from the primary membrane to a second membrane, which is the plasma fractionator. The second membrane removes high-molecular-weight molecules based on size and allows smaller molecules, such as albumin, to be filtered and returned to the patient, which allows more selective plasma removal and generally requires less albumin replacement. Double-filtration membranes are used primarily in Japan and are not approved for use in the United States.37 Plasma exchange will decrease the plasma viscosity anywhere from 30% to 50% in a single session that exchanges 1 volume of the patient’s plasma. For immunoglobulin reduction, 1 exchange can reduce the level by 60%, sufficient for reduction of viscosity to safe levels.38 Typically, only 1 session is required to reduce plasma viscosity to levels where mucosal hemorrhage will not occur. A maximum of 3 sessions are required when the viscosity exceeds 6.39 The mean length of time to perform total plasma exchange has been reported to be 91 minutes.40 In a single-center report of 260 exchange procedures, 61% were centrifugation and 39% were double-membrane filtration. They had similar efficacy in reducing viscosity. Both had the same rate of adverse reactions, with hypotension being the most common, and allergic reactions to replacement fluids the next most common. Fibrinogen levels fell by 54%, but serious bleeding was not reported.41 Toxicity leading to interruption of plasma exchange has improved over the years, falling from 2.1% to 1.3% of procedures.42 The mean frequency of moderate and severe adverse events is 5% but only 2% for hyperviscosity exchanges. A fourfold difference was found in adverse events between experienced and less-experienced centers.
When plasmapheresis reduces the IgM by ∼50%, retinopathy was reported to improve in all patients, with a measured reduction in venous diameter by an average of 15%.43 In a registry, adverse events were reported in 5.6% of plasma-exchange procedures, severe in 0.5%. Plasma exchange with filtration caused more adverse events than centrifugation, 6.4% vs 4.1%. Difficulty with access was the most common, paresthesias related to citrate and hypocalcemia in 20%, hypotension in 18%, and urticaria from replacement fluids in 9%.44 Occasionally, when significant amounts of IgM are removed from the plasma, the plasma oncotic pressure falls significantly, and free fluid extrudes through the vessel wall, which can lead to a capillary leak-like syndrome with vascular collapse.45
Hyperviscosity due to hyperleukocytosis is well described. It is generally agreed that whole-blood viscosity measurements are not required and that if the leukemia cell (blast) count is above 100 000, exchange with removal of the white cell layer is indicated. When the WBC count exceeds 100 000 cells per microliter, patients may experience signs and symptoms related to impaired tissue perfusion. This is a combination of both hyperviscosity and microvascular obstruction. Leukostasis is a significant risk in acute myeloid leukemia patients. Respiratory compromise and central nervous manifestations are the primary concern. Management of hyperleukocytosis appears to reduce the risk of early death in patients with very high WBC counts and those with monoblastic leukemia. In these situations, red cell transfusions are recommended to be withheld until the WBC count is lowered. Newly diagnosed chronic myelogenous leukemia has also been reported with hyperleukocytosis associated with headache, blurred vision, retinal hemorrhages, and a blood viscosity of 10.5 cP.46 American Society for Apheresis guidelines recommend processing ∼1.5 to 2 total blood volumes for each leukocytapheresis procedure to remove 30% to 60% of the WBCs. The benefit of processing >2 blood volumes per procedure is limited. Targeting ∼5% as the hematocrit of the WBC collection product is recommended.
Plasmapheresis can be used as primary therapy for reduction of cryoglobulin levels in cases of symptomatic essential cryoglobulinemia. Plasma exchange in the management of 5 patients with cryoglobulinemia has been reported. The procedure was carried out at room temperature with reinfusion through a blood warmer. Circulating levels of mixed cryoglobulins and monoclonal IgM cryoglobulins were more easily reduced than were IgG cryoproteins. Improvement in symptoms was associated with removal of the cryoprotein.47
For patients who have hyperviscosity, an indication for therapy, and no symptoms, rapid-acting systemic chemotherapy can reduce the serum viscosity and obviate the need for plasma exchange. Typically, rituximab-based, alkylator-based, and purine nucleoside analog-based therapies take months for a best response to be achieved and would not be considered first-line if the primary goal of therapy is rapid reduction of the viscosity level. Bortezomib-based therapies produce very rapid responses in 85% of patients, with a flare in only 11%.48 Carfilzomib-based therapy had a median time to a 50% reduction of IgM of 2.1 months.49 In most instances, an IgM reduction of as little as 25% will completely eliminate symptoms of hyperviscosity. The use of ibrutinib can produce rapid reduction in the IgM.50 In a group of patients with 1 prior therapy, the median time to a 50% IgM reduction was only 4 weeks. In multiple myeloma, there is an extensive body of literature on techniques to rapidly reduce protein levels. The focus has been in the prevention of myeloma cast nephropathy. However, the principle of rapid time to reduction in protein levels should be equally applicable to hyperviscosity. The evidence suggests that bortezomib-based triplet regimens result in the fastest decline in protein levels and should be considered essential when hyperviscosity is seen in multiple myeloma. Unfortunately, in those rare instances in which polyclonal hyperglobulinemia results in hyperviscosity, no pharmacologic technique has been shown to consistently and rapidly reduce the immunoglobulin levels. In this circumstance, plasma exchange would be recommended.
Conclusion
Plasma hyperviscosity is a rare complication of both monoclonal and polyclonal disorders associated with elevation of immunoglobulins. Plasma exchange is the therapy of choice and is relatively safe. Patients should always have confirmation of the diagnosis by measurement of the viscosity level.
Acknowledgments
This work was supported by the International Waldenström Foundation, the Amyloidosis Foundation, and Myeloma Specialized Program of Research Excellence (SPORE) P50 CA186781 from the National Institutes of Health, National Cancer Institute.
Authorship
Contribution: M.A.G. was solely responsible for manuscript preparation and data analysis; no outside preparation services were used.
Conflict-of-interest disclosure: M.A.G. reports grants and personal fees from Spectrum; personal fees from Ionis, Alnylym, Prothena, Celgene, Janssen, Annexon, Appellis, Amgen, Medscape, Physicians Education Resource, Abbvie, and Research to Practice, from Teva, outside of the submitted work; and service on the AbbVie Data Safety Monitoring Board.
Correspondence: Morie A. Gertz, Division of Hematology, Mayo Clinic, 200 SW First St, Rochester, MN 55905; e-mail: gertm@mayo.edu.