TO THE EDITOR:
In a subset of patients with acute promyelocytic leukemia (APL), t(15;17)(q22;q12) and PML-RARA fusion cannot be detected.1-3 To date, many RARA fusions have been reported with at least 12 alternative partner genes in APL.1,4-15 Here we present another novel recurrent STAT3-RARA fusion in 2 patients with APL lacking t(15;17)(q22;q12)/PML-RARA fusion.
Patient 1, a 24-year-old man, was admitted to our hospital in August 2007. Blood tests showed a hemoglobin level of 123 g/L, platelets of 89 × 109/L, and white blood cell (WBC) count of 32.3 × 109/L. Bone marrow (BM) smear showed hypercellularity with 89.3% hypergranular promyelocytes (Figure 1A). The blasts were positive for CD13 and CD33 but negative for CD117, CD34, and HLA–antigen D related (HLA-DR) by flow cytometry. However, both RT-PCR and FISH failed to detect the PML-RARA fusion gene from the BM sample of this patient (Figure 1B). Karyotype analysis revealed 45,XY,-Y[6]/46,XY[8] (Figure 1C). The patient showed no response to combination therapy with all-trans-retinoic acid (ATRA; 25 mg/m2 per day) and arsenic trioxide (10 mg per day). He then received an induction regimen of daunorubicin (40 mg/m2 per day on days 1 to 3) and cytosine arabinoside (200 mg/m2 per day on days 1 to 7) and did not achieve complete remission (CR). Therapy with a course of homoharringtonine (1 mg/m2 per day on days 1 to 10), cytosine arabinoside (10 mg/m2 subcutaneous injection once every 12 hours on days 1 to 14), and granulocyte colony-stimulating factor (150 μg daily) did lead to CR, and he then received consolidation chemotherapy with 4 courses of fludarabine (30 mg/m2 per day on days 1 to 3) plus cytosine arabinoside (2.0 g/m2 per day on days 1 to 3) and a course of mitoxantrone (8 mg/m2 per day on days 1 to 3) plus cytosine arabinoside (100 mg/m2 per day on days 1 to 7). Unfortunately, his leukemia relapsed, and he died as a result of cerebral hemorrhage in April 2010.
Identification of novel recurrent STAT3-RARA fusions in APL lacking t(15;17)(q22;q12)/PML-RARA. (A,D) May-Grünwald-Giemsa staining showing several abnormal promyelocytes in the diagnostic BM aspirate. (B,E) Interphase fluorescence in situ hybridization (FISH) using the PML-RARA dual-color dual-fusion probes revealed absence of PML-RARA. (C,F) A karyotype performed on the diagnostic BM revealed 45,XY,-Y[6]/46,XY[8] for patient 1 (P1) and 46,XY[20] for patient 2 (P2). (G) Whole-genome sequencing (WGS) analysis results revealed 1 breakpoint in intron 23 or 21 of the STAT3 gene and 2 breakpoints in intron 2 and telomere of exon 9 of the RARA gene. The 3′ region of the RARA gene (from exon 3 to exon 9) was reversed and fused in frame with the 5′ region of the STAT3 gene (from exon 1 to exon 21 or 23) in both patients. (H) Electrophoresis of reverse transcription–polymerase chain reaction (RT-PCR) products from the 2 patients showed distinct STAT3-RARA fusion transcript, and the 2 reciprocal RARA-STAT3 transcripts were also detected. (I) Partial nucleotide sequences surrounding the junctions of the 2 types of STAT3-RARA fusions. The fusion transcript from P1 was a fusion of STAT3 exon 21 and exon 3 of the RARA gene. The fusion transcript from P2 was a fusion of STAT3 exon 23 and exon 3 of RARA gene. (J) Schematic diagram of RARA, STAT3, STAT3-RARA T1, and STAT3-RARA T2 fusion proteins. The breakpoint is indicated by a red line.
Identification of novel recurrent STAT3-RARA fusions in APL lacking t(15;17)(q22;q12)/PML-RARA. (A,D) May-Grünwald-Giemsa staining showing several abnormal promyelocytes in the diagnostic BM aspirate. (B,E) Interphase fluorescence in situ hybridization (FISH) using the PML-RARA dual-color dual-fusion probes revealed absence of PML-RARA. (C,F) A karyotype performed on the diagnostic BM revealed 45,XY,-Y[6]/46,XY[8] for patient 1 (P1) and 46,XY[20] for patient 2 (P2). (G) Whole-genome sequencing (WGS) analysis results revealed 1 breakpoint in intron 23 or 21 of the STAT3 gene and 2 breakpoints in intron 2 and telomere of exon 9 of the RARA gene. The 3′ region of the RARA gene (from exon 3 to exon 9) was reversed and fused in frame with the 5′ region of the STAT3 gene (from exon 1 to exon 21 or 23) in both patients. (H) Electrophoresis of reverse transcription–polymerase chain reaction (RT-PCR) products from the 2 patients showed distinct STAT3-RARA fusion transcript, and the 2 reciprocal RARA-STAT3 transcripts were also detected. (I) Partial nucleotide sequences surrounding the junctions of the 2 types of STAT3-RARA fusions. The fusion transcript from P1 was a fusion of STAT3 exon 21 and exon 3 of the RARA gene. The fusion transcript from P2 was a fusion of STAT3 exon 23 and exon 3 of RARA gene. (J) Schematic diagram of RARA, STAT3, STAT3-RARA T1, and STAT3-RARA T2 fusion proteins. The breakpoint is indicated by a red line.
Patient 2, a 26-year-old man, was admitted to our hospital in October 2011. Blood tests showed a hemoglobin level of 73 g/L, platelets of 94 × 109/L, and WBC count of 6.6 × 109/L. BM smear showed hypercellularity with 55% hypergranular promyelocytes (Figure 1D). The blasts were positive for CD13 and CD33 but negative for CD117, CD34, and HLA-DR by flow cytometry. Both RT-PCR and FISH failed to detect the PML-RARA fusion (Figure 1E). Karyotype analysis revealed 46,XY[20] (Figure 1F). The patient was initially treated with ATRA (45 mg/m2 per day). However, BM aspiration revealed no response to ATRA after 2 weeks. He was then treated with an induction regimen of idarubicin (8 mg/m2 per day on days 1 to 3) and cytosine arabinoside (100 mg/m2 per day on days 1 to 7) and did not achieve remission. Despite receiving arsenic trioxide in combination with ATRA and several course of intensive combination chemotherapy, he never achieved CR and died as a result of cerebral hemorrhage in April 2012.
Cytogenetic and RT-PCR analyses demonstrated the absence of t(15;17)(q22;q12) and PML/RARA in both patients. To characterize the rearrangement involving the RARA gene, we performed WGS of the genomic DNA of BM samples, which revealed a recurrent STAT3-RARA fusion in both patients. WGS analysis results revealed 1 breakpoint in intron 23 or intron 21 of the STAT3 gene and 2 breakpoints in intron 2 and telomere of exon 9 of the RARA gene (Figure 1G). The 3′ region of the RARA gene (from exon 3 to exon 9) was reversed and fused in frame with the 5′ region of the STAT3 gene (from exon 1 to exon 21 or 23) in both patients (Figure 1G). RNA sequencing also confirmed the rearrangement of STAT3-RARA. For validation of this novel fusion, we performed RT-PCR and Sanger sequencing and confirmed STAT3-RARA fusion transcripts in both patients (Figure 1H-I). The main domains of RARA and STAT3 were preserved on STAT3-RARA fusion protein (Figure 1J). In the shorter transcript of STAT3-RARA, STAT3-RARA T1, the phosphorylation site of the STAT3 gene was lost; however, it was retained in the longer transcription. The reciprocal RARA-STAT3 transcripts were not detected.
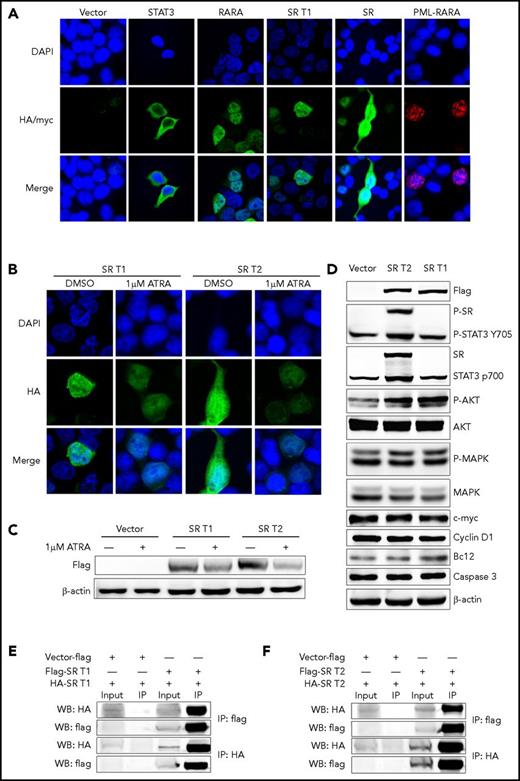
The subcellular distribution features of RARA fusion proteins are different from one another.2,13,16,17 In the present study, we demonstrated that the STAT3 protein was found in cytoplasm and the RARA in nucleus, whereas STAT3-RARA T1 was predominantly found in nucleus and STAT3-RARA-T2 located both in nucleus and cytoplasm by immunofluorescence analysis (Figure 2A). Like other RARA fusion proteins,13,15 we found that STAT3-RARA expression was downregulated by ATRA treatment by immunofluorescence analysis and western blot (Figure 2B-C). Both patients with APL showed no blast differentiation after induction therapy with ATRA alone or combined with arsenic trioxide (supplemental Figure 1, available on the Blood Web site).
Cellular location and homodimerization analyses of STAT3-RARA (SR) fusion protein. (A) Immunofluorescence analysis of 293T cells transfected with pcDNA3.1 expression plasmids of vehicle (vector), hemagglutinin (HA)-tagged STAT3, HA-tagged RARA, HA-tagged SR, and MYC-PML-RARA, respectively. HA antibody was used as primary antibody, and 4′,6-diamidino-2-phenylindole (DAPI) was used for nuclear staining. (B) ATRA downregulates SR protein expression. After transfection with pcDNA3.1 HA-tagged SR, 293T cells were treated with 1 μM of ATRA or dimethyl sulfoxide (DMSO; vehicle control) for 48 hours. The tagged protein was detected using an anti-HA antibody followed by an Alexa Fluor 488–conjugated secondary antibody. DAPI was used for nuclear staining. (C) Viral-transfected 293T cells were treated with 1 μM of ATRA or DMSO (vehicle control) for 48 hours before western blot. The tagged protein was detected using an antiflag antibody, and β-actin served as the loading control. (D) Empty-vector, flag-tagged SR T1 and T2 were expressed in 293T cells. Immunoblotting analysis of expression of flag-tagged fusions, STAT3, phosphorylated (P) STAT3, AKT, P-AKT, MAPK, P-MAPK, BCL2, c-MYC, cyclin D1, and caspase 3 was performed in 293T cell. Protein level of STAT3 targets with SR expression. (E-F) Homodimerization of SR fusions were detected by coimmunoprecipitation. Both HA-tagged SR T1 (E) and HA-tagged SR T2 (F) can be immunoprecipitated by flag antibody and vice versa. IP, immunoprecipitation.
Cellular location and homodimerization analyses of STAT3-RARA (SR) fusion protein. (A) Immunofluorescence analysis of 293T cells transfected with pcDNA3.1 expression plasmids of vehicle (vector), hemagglutinin (HA)-tagged STAT3, HA-tagged RARA, HA-tagged SR, and MYC-PML-RARA, respectively. HA antibody was used as primary antibody, and 4′,6-diamidino-2-phenylindole (DAPI) was used for nuclear staining. (B) ATRA downregulates SR protein expression. After transfection with pcDNA3.1 HA-tagged SR, 293T cells were treated with 1 μM of ATRA or dimethyl sulfoxide (DMSO; vehicle control) for 48 hours. The tagged protein was detected using an anti-HA antibody followed by an Alexa Fluor 488–conjugated secondary antibody. DAPI was used for nuclear staining. (C) Viral-transfected 293T cells were treated with 1 μM of ATRA or DMSO (vehicle control) for 48 hours before western blot. The tagged protein was detected using an antiflag antibody, and β-actin served as the loading control. (D) Empty-vector, flag-tagged SR T1 and T2 were expressed in 293T cells. Immunoblotting analysis of expression of flag-tagged fusions, STAT3, phosphorylated (P) STAT3, AKT, P-AKT, MAPK, P-MAPK, BCL2, c-MYC, cyclin D1, and caspase 3 was performed in 293T cell. Protein level of STAT3 targets with SR expression. (E-F) Homodimerization of SR fusions were detected by coimmunoprecipitation. Both HA-tagged SR T1 (E) and HA-tagged SR T2 (F) can be immunoprecipitated by flag antibody and vice versa. IP, immunoprecipitation.
STAT proteins are a family of latent cytosolic transcription factors activated by JAK tyrosine kinases and comprising 7 members: STAT1, 2, 3, 4, 5a, 5b, and 6. The STAT3 gene is located near the STAT5B gene at 17q21.2 and encodes an important transcription factor that transmits signals to the nucleus after cytokine stimulation. STAT3 mutations have been described in 30% to 40% of patients with T-cell large granular lymphocyte leukemia, leading to STAT3 pathway activation.18,19 Here, we identified the STAT3 gene as another STAT member fused with RARA in 2 men with APL by WGS and RNA sequencing, containing the same sequence of the RARA part. The main function domains of the novel STAT3-RARA fusion gene were exactly the same, except for the phosphorylation site of the STAT3 gene, which may be the reason for the slightly different protein localization. We expressed empty-vector, flag-tagged STAT3-RARA T1 and T2 in 293T cells. We investigated some downstream STAT3 targets in 293T cells, such as BCL2 and caspase 3 in cell survival, c-MYC and cyclin D1 in cell proliferation, and AKT, phosphorylated AKT, MAPK, and phosphorylated MAPK in cell signal transduction. Immunoblotting confirmed expression of both fusions, whereas STAT3 p700 and phosphorylated STAT3 Tyr705 antibody could recognize endogenous total and phosphorylated STAT3 and expressed STAT3-RARA T2 but not STAT3-RARA T1 (Figure 2D). This may have resulted from limited recognition sequence of the antibody, which cannot detect the shorter STAT3 sequence in STAT3-RARA T1. Phosphorylated AKT and BCL2 were upregulated by STAT3-RARA expression; other STAT3 targets, such as c-MYC, cyclin D1, caspase 3, and MAPK, remained unchanged (Figure 2D). These results indicate that nuclear localization of the STAT3-RARA fusion protein may result in active kinase activity and antiapoptosis ability.
It has been reported that partner-enforced RAR dimerization may be essential for APL pathogenesis.20,21 Here we investigated the homodimerization in 293T cells expressing flag-tagged and HA-tagged STAT3-RARA fusions by coimmunoprecipitation. We observed that both HA-tagged STAT3-RARA T1 and HA-tagged STAT3-RARA T2 could be immunoprecipitated by flag antibody and vice versa (Figure 2E-F). These results indicate that both types of STAT3-RARA fusions can self-associate and form homodimers.
Whereas RARA fusions with PML, NPM, NUMA, FNDC3B, and IRF2BP2 are sensitive to ATRA, PLZF-RARA and STAT5B-RARA fusions are significantly resistant.1,4-15 Here we showed that both patients with APL harboring STAT3-RARA fusions were refractory to ATRA. Tomita et al22 reported that the ATRA binding domain includes RARA exon 9, and other smaller deletions in exon 9 have been associated with secondary (acquired) ATRA resistance in APL. Kluk et al23 reported that partial loss of exon 9 of RARA may related to the resistance of STAT5B-RARA to ATRA. Because the STAT3-RARA fusions have similar breakpoint of exon 9 in RARA, we assumed that this may have contributed to the failure of both patients’ disease to respond to ATRA. Further study of the biological functions and pathogenesis of the novel STAT3-RARA fusion is needed.
In summary, we identified STAT3-RARA as another recurrent novel RARA fusion in APL. Our study highlights the importance of combining multiple molecular techniques to characterize and optimally manage APL lacking classic t(15;17)(q22;q12)/PML-RARA fusions.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Natural Science Foundation of China (81370626, 81300424, 81400112, 81400114, 81570139, 81700140), Jiangsu Province Natural Science Fund (BE2015639, BK20141201), the National Key Research and Development Program (2016YFC0902800), the Innovation Capability Development Project of Jiangsu Province (BM2015004), and Jiangsu Province Natural Science Fund (BE2015639).
Authorship
Contribution: S.C. was the principal investigator; L.Y., L.W., N.W., and T.L. performed most of the experiments; Y.X. performed clinical analysis; and S.C., D.W., and C.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suning Chen, Jiangsu Institute of Hematology, the First Affiliated Hospital of Soochow University, Shizi St 188, Suzhou 215006, P.R. China; e-mail: chensuning@suda.edu.cn.
References
Author notes
L.Y. and L.W. contributed equally to this work.

![Figure 1. Identification of novel recurrent STAT3-RARA fusions in APL lacking t(15;17)(q22;q12)/PML-RARA. (A,D) May-Grünwald-Giemsa staining showing several abnormal promyelocytes in the diagnostic BM aspirate. (B,E) Interphase fluorescence in situ hybridization (FISH) using the PML-RARA dual-color dual-fusion probes revealed absence of PML-RARA. (C,F) A karyotype performed on the diagnostic BM revealed 45,XY,-Y[6]/46,XY[8] for patient 1 (P1) and 46,XY[20] for patient 2 (P2). (G) Whole-genome sequencing (WGS) analysis results revealed 1 breakpoint in intron 23 or 21 of the STAT3 gene and 2 breakpoints in intron 2 and telomere of exon 9 of the RARA gene. The 3′ region of the RARA gene (from exon 3 to exon 9) was reversed and fused in frame with the 5′ region of the STAT3 gene (from exon 1 to exon 21 or 23) in both patients. (H) Electrophoresis of reverse transcription–polymerase chain reaction (RT-PCR) products from the 2 patients showed distinct STAT3-RARA fusion transcript, and the 2 reciprocal RARA-STAT3 transcripts were also detected. (I) Partial nucleotide sequences surrounding the junctions of the 2 types of STAT3-RARA fusions. The fusion transcript from P1 was a fusion of STAT3 exon 21 and exon 3 of the RARA gene. The fusion transcript from P2 was a fusion of STAT3 exon 23 and exon 3 of RARA gene. (J) Schematic diagram of RARA, STAT3, STAT3-RARA T1, and STAT3-RARA T2 fusion proteins. The breakpoint is indicated by a red line.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/8/10.1182_blood-2017-09-807370/4/m_blood807370f1.jpeg?Expires=1769098482&Signature=f46GmRnImW6q8dBApVAujTozh37jOpbcNela6yXmvnrpvNqSy8YOujGxONFaUhNRBJWs~uPb-L4J5RzbuUohY7QyRm7NZt5hFqM7Eun3oymAhL9EQ4yu89fnmn9E4a~B5fuX9TJu3WkVhTgLK2DXhb-ObeUXD76LoQ9jQwfkpwCyzheubdiazZqjFjvC9QOjO4OMY~8480MLrV9zGTjTQSn8C0n8ujeI2OAix~vc46x9taXqYL1TOWP2OUA8azCnYbo1T9oeKSPCmlKLhXTdcAcBpuZDf645W6NCVzdNHiMHYOfJHMsJ79PaRMCOyNn7YuTwD9X7q9s2dYzwz-fN2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)