Abstract
Combinations of proinflammatory and procoagulant reactions are the unifying principle for a variety of disorders affecting the cardiovascular system. The factor XII–driven contact system starts coagulation and inflammatory mechanisms via the intrinsic pathway of coagulation and the bradykinin-producing kallikrein-kinin system, respectively. The biochemistry of the contact system in vitro is well understood; however, its in vivo functions are just beginning to emerge. Challenging the concept of the coagulation balance, targeting factor XII or its activator polyphosphate, provides protection from thromboembolic diseases without interfering with hemostasis. This suggests that the polyphosphate/factor XII axis contributes to thrombus formation while being dispensable for hemostatic processes. In contrast to deficiency in factor XII providing safe thromboprotection, excessive FXII activity is associated with the life-threatening inflammatory disorder hereditary angioedema. The current review summarizes recent findings of the polyphosphate/factor XII–driven contact system at the intersection of procoagulant and proinflammatory disease states. Elucidating the contact system offers the exciting opportunity to develop strategies for safe interference with both thrombotic and inflammatory disorders.
Introduction
The crosstalk between coagulation and inflammation constitutes the unifying principle for a variety of disorders affecting the cardiovascular system. Elucidating the role of inflammation in coagulation and vice versa will introduce new perspectives to improve diagnostics and therapies for both disease entities, leading to improved patient care. The factor XII (FXII)–driven contact system and its activator polyphosphate (polyP) can serve as a paradigm that connects coagulation and inflammation. Here, we review novel roles of the polyP/FXII axis in thromboinflammatory diseases and its therapeutic implications when targeting its functions.
The FXII-driven contact system
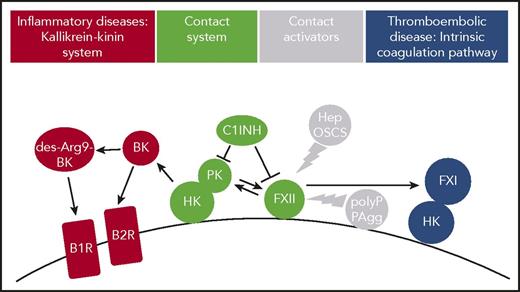
The plasma contact system is a procoagulant and proinflammatory protease cascade that is initiated by FXII, in a reaction involving high-molecular-weight kininogen (HK) and plasma kallikrein (PK) (Figure 1). FXII is the plasma zymogen form of the serine protease factor XIIa (FXIIa). FXII is activated to FXIIa following binding (“contact”) to negatively charged artificial or biologic surfaces (contact activation). Alternatively, active PK has the capacity to convert FXII zymogen to the active protease. During both contact-triggered autoactivation and PK-mediated heteroactivation, FXII zymogen undergoes limited proteolysis. FXII was first recognized as essential for surface-activated diagnostic blood coagulation assays (eg, the activated partial thromboplastin time) that are commonly used as a clinical measure of global plasma coagulation.1-3 FXII also has limited proteolytic activity, when bound to contact-activating surfaces without undergoing proteolysis. This suggests that surface binding is sufficient for inducing a conformation with some enzymatic activity that is amplified by proteolytic cleavage of the FXII zymogen form.4,5 The plasma contact system components FXII and HK assemble on cell surface proteoglycans of various cardiovascular cells, with the latter 1 bound to FXI or PK.6-9 In addition, PK also binds to proteoglycans in a HK-independent manner.10 Locally produced FXIIa initiates the intrinsic pathway of coagulation via its substrate factor XI (FXI) and leads to liberation of the proinflammatory mediator bradykinin (BK) by PK-mediated cleavage of HK.11 Serpin C1 esterase inhibitor (C1INH) is the major plasma inhibitor of FXIIa and PK. Deficiency or a dysfunctional C1INH is associated with a BK-mediated life-threatening inherited swelling disorder, hereditary angioedema (HAE) type I or II, respectively.12-14
Thromboinflammatory activities of FXII in vivo. Contact system proteins FXII, HK, and PK assemble on cell surfaces. Contact with surface-exposed nonsoluble and plasma-borne soluble contact activators, including polyP or misfolded protein aggregates (PAgg) and heparin (Hep) or oversulfated chondroitin sulfate (OSCS), respectively, leads to the active protease FXIIa. FXIIa in turn triggers thrombosis via the FXI-mediated intrinsic coagulation pathway. FXIIa also activates PK that produces BK from HK. BK activates kinin B2 receptors (B2R), whereas the BK metabolite des-Arg9 BK binds to kinin B1 receptors (B1R), both of which drive inflammation. C1INH regulates FXIIa and PK enzymatic activity.
Thromboinflammatory activities of FXII in vivo. Contact system proteins FXII, HK, and PK assemble on cell surfaces. Contact with surface-exposed nonsoluble and plasma-borne soluble contact activators, including polyP or misfolded protein aggregates (PAgg) and heparin (Hep) or oversulfated chondroitin sulfate (OSCS), respectively, leads to the active protease FXIIa. FXIIa in turn triggers thrombosis via the FXI-mediated intrinsic coagulation pathway. FXIIa also activates PK that produces BK from HK. BK activates kinin B2 receptors (B2R), whereas the BK metabolite des-Arg9 BK binds to kinin B1 receptors (B1R), both of which drive inflammation. C1INH regulates FXIIa and PK enzymatic activity.
Despite its importance for fibrin formation in vitro, for decades FXII had been considered to have no function for physiological hemostasis in vivo. This premise was based on the fact that FXII-deficient patients have a normal hemostatic capacity and do not suffer from spontaneous or injury-related increased bleeding. Thus, the contact system was dormant for 50 years but recently experienced a revival.
Twelve years with FXII-deficient mice
Our laboratory has generated the first FXII-deficient (FXII−/−) mouse model and reported in 2005 that occlusive thrombus formation in both arterial and venous beds was severely defective in these animals. However, similar to FXII-deficient individuals, the hemostatic potential of FXII−/− mice remained unaffected.15 Reconstitutions of the animals with human FXII restored the defective thrombus formation, indicating a critical function of human FXII in pathological clotting. FXII−/− mice and wild-type mice treated with FXIIa inhibitors were also protected from thromboembolic diseases, including stroke, atherothrombosis, and pulmonary embolism.16-18 The importance of FXII in thrombosis is not restricted to mice. Independent groups have confirmed the critical role that the FXII-driven intrinsic pathway of coagulation plays in thrombosis in large animals such as rats,19 rabbits,20,21 or baboons.22 Furthermore, targeting the FXIIa substrate FXI in the intrinsic pathway of coagulation has proven to be a safe therapeutic strategy in recent human studies on venous thrombosis.23 Previous work also had shown that mice deficient in the FXIIa substrate of the intrinsic pathway of coagulation FXI were protected from arterial thrombosis in an identical manner.24 Initially, defective FXI activation by tissue factor–produced thrombin (“feedback activation loop”)25,26 was proposed as the mechanistic basis underlying impaired thrombus formation in FXI−/− mice. However, the observation that occlusive thrombus formation was similarly defective in FXII−/−, FXI−/− mice, as well as in animals with combined deficiency in both clotting factors (FXII−/−/FXI−/−), indicates that FXI is mostly activated by FXIIa in pathological platelet-mediated thrombosis.18 Supporting a role of the FXII-driven contact system in thrombosis, mice with deficiencies in PK27,28 or HK29 are also protected from thrombosis. A more comprehensive overview about targeting FXII and contact system proteins in various experimental thrombosis models in animals is presented in a recent review.30
Targeting FXII/FXIIa allows for safe anticoagulation
The selective importance of FXII in pathologic thrombosis raises the exciting possibility that targeting FXII is an effective strategy for the prevention and treatment of pathologic thrombosis without the bleeding risk that is characteristic of current anticoagulants. The recombinant FXIIa inhibitor rHA-infestin-4 of insect origin largely reduced arterial and venous thrombosis and offered protection from pulmonary embolism, atherothrombosis, and ischemic stroke in rodent models without a therapy-associated increase in bleeding. Furthermore, rHA-infestin-4–treated mice were protected from experimental autoimmune encephalomyelitis31,32 and hypotonic shock in anaphylaxis.33 However, rHA-infestin-4 also inhibits plasmin and FXa at high concentrations and has immunogenic properties that limit its potential clinical applications.19,32 A fully human FXIIa neutralizing antibody, 3F7, provided protection from thrombosis in a medically meaningful setting (an experimental model of extracorporeal membrane oxygenation system in rabbits) as effective as heparin. Most importantly, unlike heparin, 3F7 did not increase bleeding.21 Consistent with the importance of the FXII-FXI axis in thrombosis, targeting activated FXI (FXIa), using an antibody against the FXIa enzymatic pocket (named DEF), interfered with thread-induced venous thrombosis in a rabbit model. In contrast to FXII, deficiency in FXI is associated with a bleeding disorder (hemophilia C). For potential interference with neutralizing FXIa-associated bleedings, a reversal agent (a human immunoglobulin G) was developed that rapidly reversed DEF antibody activity in human plasma and in vivo in rabbits.34 A summary on targeting FXI for thromboprotection is presented in an excellent review article.35 An inhibitor-independent alternative approach for FXII inhibition is based on antisense oligonucleotides–mediated interference with expression of the coagulation factor. Repetitive antisense oligonucleotides injections reduced arterial and venous thrombosis in mice to levels of <25% of normal36 and attenuated catheter-induced thrombosis in rabbits20 without increased therapy-associated bleeding. An overview on currently available experimental FXII inhibitors can be found in recent reviews.37-39
Deficiency of contact factors in humans
In contrast to the definite findings in animal models, impact of contact system deficiency states for human disease has remained less clear mostly due to small “patient” numbers and lack of matched controls.40 Human deficiency in FXII, PK, or HK is not associated with bleeding symptoms, although FXI deficiency is associated with mild bleeding, mainly in tissues with high fibrinolytic activity. Based on >500 individuals, severe FXI deficiency has been associated with lower risk for cardiovascular and venous thromboembolism events within the Ashkenazi Jewish community.41 Although FXI deficiency impairs hemostasis in this specific population, the data support a role for the intrinsic pathway protease in thrombosis in humans. To associate FXII deficiency with disease states, we are establishing a “patient” registry, including inherited severe FXII deficiencies (<10%; www.factor12.net) and invite readers to register their FXII-deficient “patients” there. The registry may help in assessing prevalence, incidence, severity, and characteristics of inflammatory, infectious, and thromboembolic diseases in the absence of contact activation. Together, these studies established an essential role of the FXII-driven intrinsic pathway of coagulation in thrombosis and led to a paradigm shift in the design of anticoagulant therapies pursuing the strategy of FXII inhibition as a safe therapeutic approach for the treatment of thrombotic diseases.
The polyP/FXII axis in thrombosis
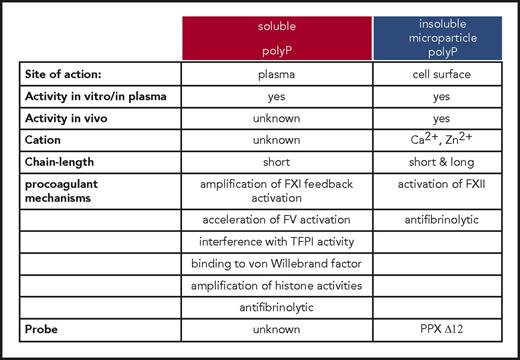
For more than half a century, it has been known that activated platelets promote coagulation in an FXII-dependent manner,42 and multiple groups have confirmed that platelets initiate coagulation in an FXII-dependent manner.43-45 The platelet-derived FXII activator, however, had remained obscure. We have identified polyP (Figure 1) as a platelet-derived FXII contact activator in vivo.18 PolyP initiates FXII contact activation in human plasma,46 with critical implications for thrombosis in vivo.18 The data extended on earlier findings, which had analyzed procoagulant activities of polyP in vitro, and found that platelet-size polyP activates FXII-dependent coagulation ex vivo.46 Oppositely, FXII-dependent plasma clotting and thrombus formation are defective in mice and humans with genetic deficiency in platelet polyP (inositol hexakisphosphate kinase 1 null [Ip6k1−/−] mice47 and Hermansky-Pudlak syndrome,18 respectively). Similar to whole platelets, membrane vesicles shed from activated platelets (microparticles) stimulate coagulation in an FXII-dependent manner.48 In addition to inducing FXII contact activation, polyP participates in an array of other procoagulant reactions involving fibrin, tissue factor pathway inhibitor, von Willebrand factor, and factors V and XI. Although polyP has the capacity to modulate these various pathways ex vivo, the in vivo relevance of the polymer in these mechanisms has remained unknown.49,50 PolyP procoagulant activity increases with chain length. Plasma-soluble polymers have a mean chain length of 60 to 100 and have only limited capacity to trigger FXII contact activation51 ; however, they strongly interfere with tissue factor pathway inhibitor activities in buffer systems.52 The fact that activated platelets potently activate FXII suggests that distinct polyP pools exist in platelets (Figure 2). Platelets store polyP together with high concentrations of calcium ions (>2 molar) in intracellular organelles (dense granules), and released polyP is complexed with Ca2+ ions.53 When Ca2+-polyP nanoparticles are formed under physiological conditions, these are stable for hours.54 A small fraction of platelet polyP only is secreted into the supernatant upon activation; however, the vast majority of platelet polyP is retained on procoagulant platelet cell surface upon secretion in the form of Ca2+-rich nanoparticles.55 These polyP nanoparticles potently initiate FXII contact activation and drive coagulation via the intrinsic pathway on the platelet surface.55 Similar to kaolin (a silicate that is commonly used to trigger FXII activation in the activated partial thromboplastin time clotting assay), Ca2+-polyP nanoparticles are not soluble and accumulate on the platelet membranes via unidentified cell surface binding sites.56 Ca2+-polyP nanoparticles appear to represent the natural kaolin that initiates the contact system on cardiovascular surfaces. Similar to polyP nanoparticles, short-chain polyP that is conjugated to colloidal gold nanoparticles activates FXII to a similar extent as compared with long-chain polyP.57 Consistently, localization onto particles increases procoagulant activity of short-chain polyP even under flow conditions.58 The procoagulant properties of insoluble polyP packed in nanoparticles largely differ from those of molecularly dissolved soluble molecules. FXII-activating properties of polymers in nanoparticle form largely exceed that of dispersed polyP in solution.54,56 The formation of Ca2+-polyP aggregates with increased capacity for inducing FXII contact activation argues against a decisive role of polymer chain length in regulating polyP activity in vivo. Synthetic long-chain polyP binds to the platelet surface, whereas short-chain polyP does not.59 This could indicate that polyP microparticles on the platelet surface contain longer polymers than are found in the supernatant of activated platelets or condensed aggregated short-chain molecules (Figure 2). Taken together, the polyP/FXII-driven coagulation has a critical function for thrombosis albeit does not contribute to hemostatic processes (Figure 3). The findings with polyP/FXII also suggest that the classical concept of a coagulation balance with hemostasis and thrombosis presenting the opposite poles has some exceptions.
Activities and functions of polyP in the coagulation system. Two different pools of polyP that differ in their biophysical properties and procoagulant mechanisms, respectively, operate in the coagulation cascade. Insoluble long-chain polyP and polymers condensed in microparticles initiate FXII contact activation on cell surfaces. Although mechanisms driven by insoluble polyP microparticles have been analyzed in vivo, functions of soluble/short-chain polymers are based on in vitro studies.
Activities and functions of polyP in the coagulation system. Two different pools of polyP that differ in their biophysical properties and procoagulant mechanisms, respectively, operate in the coagulation cascade. Insoluble long-chain polyP and polymers condensed in microparticles initiate FXII contact activation on cell surfaces. Although mechanisms driven by insoluble polyP microparticles have been analyzed in vivo, functions of soluble/short-chain polymers are based on in vitro studies.
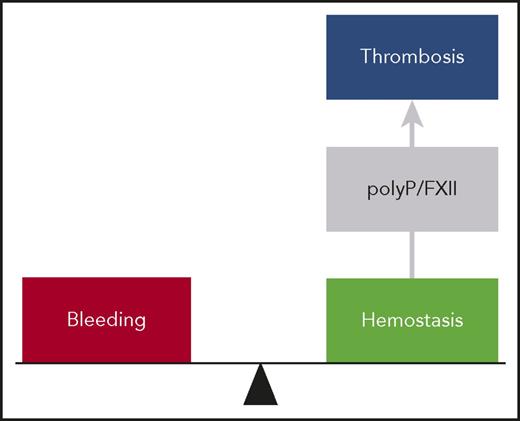
The polyP/FXII axis in thrombosis. At a site of vessel injury, tissue factor–initiated coagulation mechanisms mediate hemostasis, and deficiency in these pathways results in bleeding. In thrombosis, activated platelets expose polyP nanoparticles that induce FXII contact activation driving coagulation within the thrombus. The polyP/FXII specifically contributes to pathologic coagulation, and targeting polyP or FXII interferes with thrombosis but has no impact on hemostasis, suggesting use of this strategy for safe thromboprotection.
The polyP/FXII axis in thrombosis. At a site of vessel injury, tissue factor–initiated coagulation mechanisms mediate hemostasis, and deficiency in these pathways results in bleeding. In thrombosis, activated platelets expose polyP nanoparticles that induce FXII contact activation driving coagulation within the thrombus. The polyP/FXII specifically contributes to pathologic coagulation, and targeting polyP or FXII interferes with thrombosis but has no impact on hemostasis, suggesting use of this strategy for safe thromboprotection.
Based on the potent procoagulant activities of polyP, the polymer has emerged as a novel target for anticoagulant drugs. Several strategies to achieve this aim have been developed. First, recombinant salivary proteins of the African sand fly (PdSP15) bind polyP and interfere with FXII contact system–driven clotting and inflammation. However, PdSP15 binds to various other polyanions, such as silica, limiting its potential therapeutic application.60 Second, cationic polyethylenimine and polyamidoamine dendrimers bind polyP and attenuate thrombosis in mouse models.61,62 Unfortunately, polyethylenimine and polyamidoamine at a concentration required to provide thromboprotection is cytotoxic to cultured cells. Third, the crown ether-based universal heparin reversal agents (UHRAs that originally were developed as heparin antidotes) are nontoxic and inhibit polyP procoagulant activity. However, UHRAs also interfere with tissue factor–initiated fibrin formation,63 and UHRA infusions lead to bleeding in murine models,63 involving charge-driven interference with factor V activation. Finally, recombinant Escherichia coli exopolyphosphatase (PPX, an enzyme that specifically degrades polyP of chain length >35 phosphate units) mutants even at the highest concentrations tested selectively targeted polyP. PolyP binding by truncated mutant PPX_Δ12 or degradation by full-size PPX, respectively, almost completely blunted fibrin formation on activated platelets and ablated arterial and venous thrombosis in mouse models in an FXIIa-dependent manner. Supporting the notion that polyP functions via FXII contact activation in vivo, specific neutralization of polyP interfered with thrombosis but did not impair the hemostatic capacity of treated animals, reproducing the thromboprotective phenotype of FXII-deficient animals. Targeting polyP in the circulation and at platelet surfaces for interference with thrombosis and inflammation in vivo is a matter of ongoing research. The nucleotide RNA is negatively charged and has the capacity for inducing FXII contact activation ex vivo.64 Extracellular RNA has been considered to promote thrombosis in vivo in an FXII-dependent manner. This notion is based on the observation that infusion of RNase (an enzyme that degrades RNA) interferes with arterial thrombosis in a murine arterial thrombosis model.65 However, RNase also readily hydrolyzes the phosphoanhydride bonds in the polyP backbone and efficiently degrades the polymer, offering an alternative explanation for the thromboprotective effects conferred by the enzyme.55 Targeting both polyP and FXII reduces mechanical stability of the clot structure within the thrombus in vitro by interference with fibrin fiber formation.18,46 Oppositely, interference with FXIIa-driven coagulation increases embolization events following atherothrombosis, confirming a role of polyP/FXII for thrombus propagation distant from the vessel wall but not in coagulation mechanisms at the site of vascular injury.17,66
The importance of polyP in thrombosis is not restricted to platelets. Cancer cells and cancer-derived microparticles expose polyP on their surface that initiates pathologic clotting in an FXII-dependent manner in murine venous thrombosis models and patients with malignancies,67 suggesting that targeting polyP could be a novel therapy in a variety of disease states associated with increased thrombotic risk. PolyP is not stable, and the polymer is degraded with a 2-hour half-life in plasma.46 Furthermore, purified polyP loses its procoagulant activity during storage.68 The polyP/FXII pathway operates independently of tissue factor–mediated coagulation and triggers thrombin formation even when the tissue factor pathway is inhibited.68 Oppositely, FXII−/− mice are protected from tissue factor–infusion triggered thrombosis. Independent of coagulation, polyP potently triggers inflammatory activities in rodent models. FXIIa that is generated following binding to polyP activates the kallikrein-kinin system, resulting in BK generation in vitro and BK-driven edema in vivo.30 Furthermore, polyP modulates the complement system69 and can activate the mammalian target of rapamycin in breast cancer cells and thus stimulate mitogenic signaling pathways.70 The polymer also forms high-affinity complexes with high mobility group box 1 (HMGB1) and histone 471 ; both proteins are increased in serum/plasma of septic patients.72 PolyP binding to these proteins amplifies proinflammatory signaling in endothelial cells through interaction and activation of 2 cell surface receptors, RAGE and P2Y1,71 leading to disruption of endothelial barriers by mammalian target of rapamycin–dependent pathways.73 Furthermore, platelet polyP binding to histones initiates thrombin formation independent of contact activation.74 To date, little is known about polyP biosynthesis and regulation in mammalian systems; however, the recently established fluorescence-activated cell sorting assay to measure polyP in patient samples based on a recombinant polyP-specific probe (PPX_Δ12) may open the avenue to establish the polymer as a novel biomarker.61
The contact system as mediator of inflammation
Because of its original identification as part of the coagulation mechanism, the inflammatory branch of the contact system has long been underappreciated. However, quantitative and qualitative C1INH deficiency (C1INH-HAE; also termed type I and II HAE) results in aggressive attacks of tissue swelling in the rare disease HAE. These swelling attacks are caused by excessive production of BK as a result of a hyperactive contact activation system.11 BK acts on kinin receptors that are constitutively present on the endothelium (kinin B2 receptors) and can be expressed by leukocytes during inflammatory reactions (kinin B1 receptors). Kinin receptor signaling (among others) increases vascular leakage. BK has a half-life of seconds because of the rapid breakdown by kininases (enzymes that break down kinins) in blood and tissue. Angiotensin-converting enzyme is an important kininase and angiotensin-converting enzyme inhibitor use is known to cause angioedema (without C1INH deficiency) as a rare side effect. Murine models of C1INH deficiency have recapitulated the phenotype of excessive BK-mediated vascular leakage that characterizes HAE.75,76 Attacks of angioedema can occur throughout the body and are often seen in mucosal tissues, including the face and gastrointestinal tract as well as genitals and extremities. When the upper airways are threatened, it can have life-threatening consequences. Although attacks can travel from 1 site in the body to another (sometimes 2 sites are simultaneously affected),77 there is a remarkable restriction to the extent of vascular leakage. In other words, there is little evidence for a systemically increased vascular permeability as blood pressure remains largely normal. There are 2 complementary explanations for the restricted localization of tissue swelling: (1) The affected tissues are “primed” by locals threats (injury or infection) leading to kinin B1 receptor expression. Systemic BK production (which should happen constitutively) becomes locally visible as a result.78 (2) Analogous to blood coagulation, factors of the contact system assemble on the vascular endothelium,7 or in the vicinity of activated leukocytes and mast cells. BK is locally produced and acts on nearby tissue in a paracrine manner.8 Kininases prevent systemic action of this potent proinflammatory peptide. A particularly interesting feature of HAE is that there is a striking lack of prothrombotic features, suggesting that BK production can be initiated in the absence of (clinically significant) coagulation.79 The mechanisms that drive this mechanistic uncoupling of FXIIa-driven thrombosis and inflammation deserve further scientific attention. All FXII contact activators identified so far either trigger both coagulation and inflammation or inflammation alone but not specifically coagulation without inflammation.80 BK-driven tissue swelling attacks can also take place when C1INH levels are normal and with equally dangerous consequences. In a subset of HAE patients, the disease is not caused by C1INH deficiency, but by gain-of-function mutations in the F12 gene (encodes FXII) instead (FXII-HAE/type III HAE81 ). The clinical phenotype of these patients during attacks is very comparable with those that have C1INH-dependent HAE, although the estrogen dependence of the disease is more prominent.82 Furthermore, excessive BK production is observed in patients with angioedema of unknown origin,83 and BK-driven edema accompanies severe allergic reactions.33 These observations indicate that imbalances in the plasma contact system have pathological consequences. The associated spontaneous pathology has inflammatory features, rather than thrombotic features.
We recently performed detailed investigation of the FXII-HAE mutations, all of which are located in the proline-rich region that is unique to FXII. We first discovered that the mutations cause the production of a functional FXII protein that has a glycosylation defect.79 This reduces the net charge of the protein, promoting association to the contact system-activating polymer dextran sulfate. In vivo expression of FXII-HAE mutants in mice results in an increased propensity to develop edema in a provocation model. We subsequently identified that these mutations also render the FXII molecule sensitive to activating cleavage by plasmin.84 Although not considered part of the contact system in vitro, plasmin has a known capacity for FXII activation.85 In addition, recent studies indicate that soluble polyP enhances the plasminogen activator function of FXIIa.86 Furthermore, BK signaling contributes to blood-brain barrier permeability in hyperfibrinolytic states,87 and oppositely, antifibrinolytic agents have therapeutic value in HAE and idiopathic angioedema.88 Together, this implicates plasmin as an active player in activation of the contact system during BK production in HAE. More recent studies indicate that the interaction between the fibrinolytic system and the contact system extends beyond HAE. Brain edema is a clinically relevant side effect of thrombolytic therapy, but is considered a necessary evil to clear obstructive thrombi. It was recently demonstrated that BK is an essential player in the development of brain edema that follows plasmin activity.87 Other studies demonstrated the involvement of PK in the development of thrombolysis-associated brain edema89 and confirmed the exciting possibility that targeting the contact system eliminates this dangerous side effect of this essential therapy.
Conclusion
In this review, we provided an overview on the contact system and its activators in thrombosis and BK-mediated inflammatory reactions. Since the original discovery that FXII is essential for “pathological” thrombosis in 2005, the contact system and its activator polyP have been recognized to critically contribute to thromboembolic and inflammatory disorders. Targeting FXIIa and polyP interferes with the polyP/FXII axis, providing safe thromboprotection with additional anti-inflammatory activities. Because polyP/FXII inhibition potently protects from thrombosis without increasing bleeding risk, the proposed studies have the potential to improve the benefit-to-risk profile of anticoagulant therapy in comparison with currently available agents (heparins, vitamin K antagonists, factor Xa inhibitors, or thrombin inhibitors). We propose that therapeutic targeting of FXII/FXIIa has an optimal safety profile for contact system–driven thrombosis. Furthermore, therapeutic interference with the contact system factors has the additional benefit of having anti-inflammatory90 and healing91 potential. Targeting these combined features appears as the ultimate strategy to interfere with thromboinflammation. Furthermore, these studies will lay the foundation for a more complete understanding of how targeting polyP and FXII may serve as a key strategy to improving infectious, autoimmune, and allergic diseases.
Acknowledgments
The authors thank all the researchers that they had the pleasure to collaborate with in the last 12 years of exciting research with FXII.
This work was supported in part by grants from the German Research Society (SFB877, TP A11, SFB841, TP B8), and a European Research Council grant (ERC-StG-2012-311575_F-12) (T.R.). C.M. gratefully acknowledges financial support from the Landsteiner Foundation for Blood Transfusion Research and the Netherlands Thrombosis Foundation.
Authorship
Contribution: T.R. and C.M. wrote the review and discussed its contents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Renné, Institute of Clinical Chemistry and Laboratory Medicine, University Medical Center Hamburg-Eppendorf, Martinistraße 52, D-20246 Hamburg, Germany; e-mail: thomas@renne.net.