TO THE EDITOR:
Drug-induced immune thrombocytopenia (DITP) is caused by drug-dependent platelet-reactive antibodies DDAbs) that induce platelet destruction when a drug is taken for which the antibody is specific.1-5 The widely used cancer drug oxaliplatin is a common trigger for DITP.6-8 We recently encountered 2 patients who experienced severe thrombocytopenia after exposure to oxaliplatin and had recurrences when first oxaliplatin and then other drugs were omitted from treatment regimens, which suggests that multiple drug-dependent antibodies might be present. Here we describe studies that confirm this possibility and provide evidence that patients treated with oxaliplatin are unusually prone to producing multiple DDAbs specific for drugs to which they are exposed.
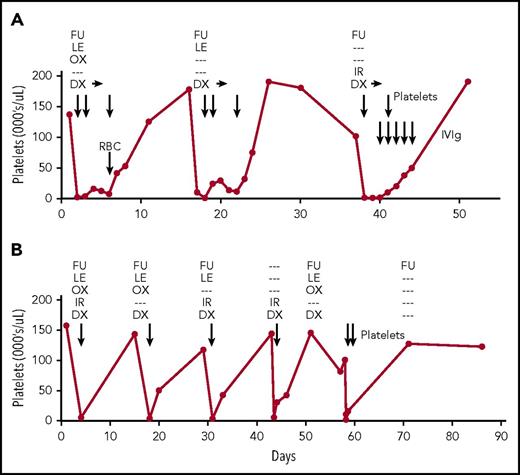
Patient 1 (Figure 1A) was a 74-year-old woman with gastric carcinoma who was receiving fortnightly infusional fluorouracil (FU), leucovorin, and oxaliplatin (FOLFOX) and bevacizumab with adjunctive dexamethasone and palonosetron. After the twentieth treatment cycle, platelets dropped from 130 000/μL to an undetectable level and hemoglobin decreased from 10.8 g/dL to 7.5 g/dL. A direct Coombs test was weakly positive for immunoglobulin G. Platelets, red blood cells, and dexamethasone 40 mg once per day for 3 days were administered, but platelet counts remained in the single digits and started to increase only after dexamethasone was discontinued. Oxaliplatin was omitted from the next cycle, but platelets dropped from 175 000/μL to 5000/μL and remained low for several days. Oxaliplatin and leucovorin were then omitted and irinotecan was added, but platelets again dropped from 101 000/μL to 10 000/μL. After treatment with dexamethasone, platelets, and intravenous immunoglobulin G, platelets returned to normal. She subsequently received 13 cycles of docetaxel, FU, and prednisone without affecting platelet counts.
Clinical course of patients 1 and 2. (A-B) Medications administered during cycles of treatment are shown at the top of each panel. Dexamethasone (DX) was continued for 3 days after each episode of thrombocytopenia in patient 1 (horizontal arrows). Platelet and red blood cell (RBC) transfusions and intravenous immunoglobulin (IVIg) infusions are indicated by vertical arrows. Days (x-axis) are numbered from the first cycle of chemotherapy that was followed by an episode of thrombocytopenia. IR, irinotecan; LE, leucovorin; OX, oxaliplatin.
Clinical course of patients 1 and 2. (A-B) Medications administered during cycles of treatment are shown at the top of each panel. Dexamethasone (DX) was continued for 3 days after each episode of thrombocytopenia in patient 1 (horizontal arrows). Platelet and red blood cell (RBC) transfusions and intravenous immunoglobulin (IVIg) infusions are indicated by vertical arrows. Days (x-axis) are numbered from the first cycle of chemotherapy that was followed by an episode of thrombocytopenia. IR, irinotecan; LE, leucovorin; OX, oxaliplatin.
Patient 2 (Figure 1B) was a 53-year-old man with colon cancer who was receiving fortnightly FU, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) and adjunctive dexamethasone and ondansetron. After the eighteenth cycle, platelets dropped from 157 000/μL to 5000/µL. After transfusions, platelets gradually returned to normal. Irinotecan, a known cause of DITP,9 was omitted from the next cycle, but platelets dropped from 143 000/µL to 4000/µL and later returned to 118 000/µL. Further episodes of profound thrombocytopenia occurred after treatments in which oxaliplatin was omitted, then irinotecan and dexamethasone alone were given and, finally, irinotecan alone was omitted. He subsequently received FU alone, FU and gemcitabine, FU-gemcitabine-cisplatin, and FU-bevacizumab-methotrexate without affecting platelet counts.
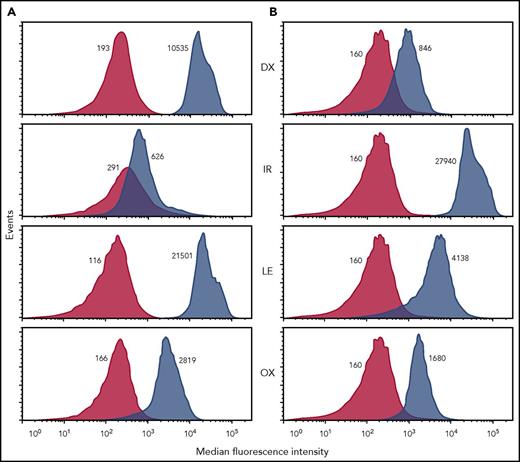
By using a flow cytometric assay,2,10 patient samples were tested with each of the chemotherapeutic drugs and adjuncts used in treatment and were found to contain DDAbs specific for oxaliplatin, leucovorin, dexamethasone, and irinotecan (Figure 2). Weak DDAbs specific for the antiemetic ondansetron were also found (not shown). The strongest antibodies were specific for dexamethasone and leucovorin in patient 1 and irinotecan in patient 2. In a platelet antibody bead array assay,11 it was found that dexamethasone- and leucovorin-dependent antibodies from patient 1 and the irinotecan-dependent antibody from patient 2 recognized αIIb/β3 integrin (GPIIb/IIIa) and the oxaliplatin-dependent antibody from patient 1 recognized GPIb/IX (supplemental Figure 1, available on the Blood Web site). Other DDAb targets were not identified. Patient sera were then absorbed with platelets in the presence of 3 drugs that produced the strongest reactions in flow cytometry and were dialyzed to remove the drug. In each case, antibody specific for the drug used in absorption was completely removed without affecting other DDAbs (supplemental Figure 2), indicating that multiple distinct DDAbs were present rather than a single antibody that cross-reacted with multiple drugs.
Drug-dependent, platelet-reactive immunoglobulin G antibodies specific for dexamethasone, irinotecan, leucovorin, and oxaliplatin were detected in both patient 1 and patient 2. Figure shows representative flow cytometric studies in which patient serum was incubated with normal group O platelets in the presence (blue peaks) and absence (red peaks) of drug 1.0 mg/mL. After washing, platelet-bound immunoglobulin G (IgG) was detected with an IgG-specific secondary antibody labeled with fluorescein isothiocyanate. Median fluorescence intensity (MFI) values are shown adjacent to each peak. A positive test result is one in which the ratio of the MFI values obtained with and without drug exceeds 1.50. MFI values obtained when normal serum was substituted for patient serum did not differ significantly from those obtained with patient serum in the absence of drug (not shown). Patient serum was also screened for DDAbs specific for ondansetron and palonosetron (antiemetics), and diphenhydramine (antihistamine), agents commonly used to reduce adverse effects in patients given combination chemotherapy for cancer. Both patients were found to have weak but reproducible DDAbs specific for ondansetron. Because patient 1 was not given ondansetron during the cycles of treatment shown in Figure 1, it is likely that the ondansetron-specific DDAb resulted from exposure to this drug during an earlier treatment cycle.
Drug-dependent, platelet-reactive immunoglobulin G antibodies specific for dexamethasone, irinotecan, leucovorin, and oxaliplatin were detected in both patient 1 and patient 2. Figure shows representative flow cytometric studies in which patient serum was incubated with normal group O platelets in the presence (blue peaks) and absence (red peaks) of drug 1.0 mg/mL. After washing, platelet-bound immunoglobulin G (IgG) was detected with an IgG-specific secondary antibody labeled with fluorescein isothiocyanate. Median fluorescence intensity (MFI) values are shown adjacent to each peak. A positive test result is one in which the ratio of the MFI values obtained with and without drug exceeds 1.50. MFI values obtained when normal serum was substituted for patient serum did not differ significantly from those obtained with patient serum in the absence of drug (not shown). Patient serum was also screened for DDAbs specific for ondansetron and palonosetron (antiemetics), and diphenhydramine (antihistamine), agents commonly used to reduce adverse effects in patients given combination chemotherapy for cancer. Both patients were found to have weak but reproducible DDAbs specific for ondansetron. Because patient 1 was not given ondansetron during the cycles of treatment shown in Figure 1, it is likely that the ondansetron-specific DDAb resulted from exposure to this drug during an earlier treatment cycle.
We next studied 53 archived serum samples from patients referred for testing because their symptoms suggested oxaliplatin-induced thrombocytopenia. Thirty samples had tested positive for oxaliplatin-dependent DDAbs and 23 had tested negative. Seven of the 30 patients with oxaliplatin-specific DDAbs were known to have experienced acute thrombocytopenia (median platelet nadir, 5000/μL) after 10 or more cycles of chemotherapy. Each serum sample was tested by flow cytometry for DDAbs specific for drugs commonly used in chemotherapeutic regimens that include oxaliplatin. As shown in supplemental Table 1, serum samples from 14 (47%) of the 30 patients with oxaliplatin-dependent DDAbs contained 34 other DDAbs specific for leucovorin (12), irinotecan (2), dexamethasone (7), ondansetron (7) diphenhydramine (2), and palonosetron (4), whereas only 3 such antibodies were found in 23 sera that lacked oxaliplatin-dependent DDAbs.
Identification of multiple DDAbs in each patient and stability of platelet counts when treatment regimens were modified to avoid drugs for which antibodies were specific makes it highly likely that the various DDAbs identified were responsible for recurrent thrombocytopenic episodes in the 2 index patients. Persistence of profound thrombocytopenia when dexamethasone was continued for 3 days (Figure 1A) provides particularly strong evidence that the potent dexamethasone-specific antibody in patient 1 was capable of causing drug-dependent thrombocytopenia. Findings from a retrospective study of serum samples from 53 patients with symptoms that may have indicated oxaliplatin-induced immune thrombocytopenia suggest that the 2 index patients were not unique, because about half the 30 patients who made an oxaliplatin-dependent DDAb were found to have DDAbs specific for 1 or more other drugs commonly used with oxaliplatin in treatment regimens. DDAbs specific for leucovorin, dexamethasone, ondansetron, and diphenhydramine identified in this group were not implicated previously as causes of DITP2-5,12,13 and are not listed in an extensive online registry of drugs implicated in DITP maintained by J.N. George and associates (http://www.ouhsc.edu/platelets/). Identification of multiple distinct DDAbs in the index patients and in archived serum samples from patients who made oxaliplatin-specific DDAbs is remarkable, because the literature contains only 2 examples of a patient who made even 2 DDAbs simultaneously.13,14 The 7 drugs identified as triggers for DDabs do not have obvious structural similarities (supplemental Figure 3). Together, the findings suggest that patients who produce an oxaliplatin-dependent DDAb have a remarkable tendency to produce DDAbs specific for other drugs being administered.
After being infused, oxaliplatin is converted to reactive metabolites capable of linking covalently to proteins, DNA, and other macromolecules.15 The resulting adducts could conceivably be recognized as foreign by the immune system, which would account for a spectrum of oxaliplatin adverse effects termed “oxaliplatin immune-induced syndrome” that occurs after 10 to 20 treatment cycles.15 As in patient 1, who had immune thrombocytopenia and hemolytic anemia, oxaliplatin-treated patients sometimes experience more than 1 of these immunologic complications.15-17 It has been suggested that antibodies induced by oxaliplatin cause blood cell destruction by recognizing drug linked to membrane glycoproteins.18,19 However, oxaliplatin-specific platelet antibodies bind strongly to their targets when the drug is present at pharmacologic levels of 2 to 4 μg/mL, an amount far too low to create a target for antibody on the platelet surface.6,20 Recent articles provide evidence that drugs promote binding of DDAbs to platelets not by binding to and modifying a target on the cell membrane but through a high-affinity interaction between drug and the complementarity-determining region of antibody that reconfigures the complementarity-determining region in such a way that it acquires specificity for a site on a platelet glycoprotein.21,22 Strong binding of oxaliplatin-induced antibodies to platelets at very low concentrations of soluble drug is consistent with this possibility.6,20
None of these considerations provide an explanation for what our findings suggest is a remarkable tendency of patients who become sensitized to oxaliplatin to produce DDAbs specific for other medications included in their treatment. Experience with the patients described in this article indicates that these extra DDAbs can produce severe thrombocytopenia upon administration of the drug for which they are specific. It is important that physicians caring for patients who may have oxaliplatin-induced thrombocytopenia appreciate that other agents being used in treatment simultaneously can also contribute to platelet destruction.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by grant HL-13629 from the National Heart, Lung, and Blood Institute, National Institutes of Health.
All studies involving human subjects were approved by the Institutional Review Board of the Medical College of Wisconsin.
Authorship
Contribution: R.H.A. oversaw all aspects of the research, facilitated communications between investigators, and wrote the manuscript; B.R.C. designed and oversaw serologic studies, interpreted findings, and facilitated communications between investigators; N.P., J.L., and S.C. provided medical care, obtained serum samples, and summarized clinical and laboratory findings in patient 1; Y.-M.S.H., R.A.D., and M.S.S. provided medical care, obtained serum samples, and summarized clinical and laboratory findings in patient 2; K.Z. performed serologic studies; D.W.B. designed and oversaw serologic studies and maintained the serum sample archive; and all authors reviewed several versions of the manuscript and suggested edits.
Conflict-of-interest disclosure: The authors declare no competing financial interests
Correspondence: Richard H. Aster, BloodCenter of Wisconsin, Medical College of Wisconsin, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: richard.aster@bcw.edu.