Key Points
HSP47+ myofibroblasts are accumulated in the fibrotic lesions of chronic GVHD and promote fibrosis in a CSF-1R+ macrophage-dependent manner.
Vitamin A–coupled liposomes containing HSP47 siRNA abrogate HSP47 expression in myofibroblasts and ameliorate fibrosis in chronic GVHD.
Abstract
Chronic graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (SCT) is characterized by multiorgan fibrosis and profoundly affects the quality of life of transplant survivors. Heat shock protein 47 (HSP47), a collagen-specific molecular chaperone, plays a critical role in collagen synthesis in myofibroblasts. We explored the role of HSP47 in the fibrotic process of cutaneous chronic GVHD in mice. Immunohistochemical analysis showed massive fibrosis with elevated amounts of collagen deposits and accumulation of F4/80+ macrophages, as well as myofibroblasts expressing HSP47 and retinol-binding protein 1 in the skin after allogeneic SCT. Repeated injection of anti–colony-stimulating factor (CSF-1) receptor-blocking antibodies significantly reduced HSP47+ myofibroblasts in the skin, indicating a macrophage-dependent accumulation of myofibroblasts. Vitamin A-coupled liposomes carrying HSP47 small interfering RNA (siRNA) (VA-lip HSP47) delivered HSP47 siRNA to cells expressing vitamin A receptors and knocked down their HSP47 in vitro. Intravenously injected VA-lip HSP47 were specifically distributed to skin fibrotic lesions and did not affect collagen synthesis in healthy skin. VA-lip HSP47 knocked down HSP47 expression in myofibroblasts and significantly reduced collagen deposition without inducing systemic immunosuppression. It also abrogated fibrosis in the salivary glands. These results highlight a cascade of fibrosis in chronic GVHD; macrophage production of transforming growth factor β mediates fibroblast differentiation to HSP47+ myofibroblasts that produce collagen. VA-lip HSP47 represent a novel strategy to modulate fibrosis in chronic GVHD by targeting HSP47+ myofibroblasts without inducing immunosuppression.
Introduction
Allogeneic hematopoietic stem cell transplantation (SCT) is a curative therapy for hematologic malignancies, but donor T-cell–mediated graft-versus-host disease (GVHD) hampers wider application of SCT. Although recent progress in allogeneic SCT has resulted in increased long-term survival time in patients who have undergone SCT,1 the incidence of chronic GVHD has increased in recent years.2 Chronic GVHD involves various organs such as the skin, liver, lung, salivary glands, and hematopoietic system and profoundly affects the quality of life of long-term survivors of SCT.3 Prolonged inflammatory responses after SCT initiate the fibrotic cascade; fibrosis of the epithelial and mucosal tissues is a cardinal feature of chronic GVHD.4 Systemic administration of corticosteroids remains the standard therapy for chronic GVHD, but long-term use of corticosteroids is associated with poor outcomes due to increased risks of infection and other adverse effects.5,6 Development of novel treatment strategies for chronic GVHD without induction of immunosuppression is an urgent unmet medical need.
Because organ fibrosis is the main manifestation of chronic GVHD, antifibrotic therapy is a promising strategy to treat chronic GVHD without inducing immune suppression. Organ fibrosis is characterized by increased deposition of collagen fibers secreted by myofibroblasts derived from fibroblasts.7 Heat shock protein 47 (HSP47) is a stress protein with a unique character as a molecular chaperone that specifically binds to procollagen in the endoplasmic reticulum and plays a critical role in collagen synthesis and secretion.8,9 In the absence of HSP47 activity, collagen fibers are misfolded and are accumulated in myofibroblasts, resulting in endoplasmic reticulum stress and subsequent apoptosis of myofibroblasts.9 Thus, HSP47 is a promising target to suppress fibrosis.
Vitamin A-coupled liposomes (VA-lip) recently have been developed to deliver HSP47 small interfering RNA (siRNA) specifically to pathogenic myofibroblasts.10 It has been shown that VA-lip containing HSP47 siRNA (VA-lip HSP47) target myofibroblasts and mitigate fibrosis in experimental models of liver cirrhosis, pancreatic fibrosis with chronic pancreatitis, and bleomycin-induced lung fibrosis.10,11 In this study, we evaluated the role of HSP47 in fibrotic chronic GVHD and if VA-lip HSP47 could target skin myofibroblasts and ameliorate fibrosis in experimental chronic GVHD.
Materials and methods
Mice
Female C57BL/6 (B6, H-2b) and BALB/c (H-2d) mice were purchased from Charles River Japan (Yokohama, Japan). B10.D2 (H-2d) mice were purchased from Japan SLC (Shizuoka, Japan). We performed all animal experiments under the auspices of the Institutional Animal Care and Research Advisory Committee of Hokkaido University.
SCT
Bone marrow transplantation (BMT) was performed as described previously.12 Briefly, BALB/c mice received a single dose of 6-Gy total-body irradiation (TBI) followed by intravenous injection of 8 × 106 BM cells plus 2.5 × 107 splenocytes from minor histocompatibility antigen–mismatched B10.D2 or syngeneic BALB/c donors on day 0. We performed SCT using granulocyte colony-stimulating factor (G-CSF)–mobilized splenocytes as described previously.13 After receiving 12 Gy of TBI on day −1, B6 recipients were transplanted on day 0 with 20 × 106 splenocytes harvested from syngeneic B6 or allogeneic BALB/c donors injected with 10 μg of G-CSF (Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan) daily from day −6 to day −1. Mice were maintained in specific pathogen-free conditions and received normal chow and autoclaved hyperchlorinated water. To deplete macrophages, anti-CSF1 receptor monoclonal antibodies (αCSF1R mAbs, clone: AFS98) were purified from hybridomas and intraperitoneally administered at 0.5 mg/body 3 times per week from day +7 after BMT, as described previously.14
Evaluation of chronic GVHD
After BMT, survival was monitored daily. For histological analysis, skin samples were fixed in 4% paraformaldehyde and then embedded in paraffin. Paraffin sections (7 μm thick) of the tissues were stained with hematoxylin and eosin (H&E) and Masson trichrome (MT). We acquired images at room temperature using an all-in-one fluorescence microscope BZ-9000 (Keyence, Osaka, Japan) with a 10×/0.30 NA objective lens. Skin thickness was measured as the distance between the bottom of epidermis to the top of the fat layer using the ImageJ software (National Institutes of Health, Bethesda, MD; https://imagej.nih.gov/ij/), as described previously.15 We histopathologically assessed chronic GVHD using a semiquantitative scoring system based on dermal fibrosis, fat loss, inflammation, epidermal interface changes, and follicular dropout (0-2 for each category, the maximum score was 10), as reported previously.12
Bleomycin-induced skin fibrosis model
B6 mice were subcutaneously injected with 100 μg/body of bleomycin (Nippon Kayaku, Tokyo, Japan) to their shaved back skin daily for 21 days.
VA-lip HSP47
VA-lip containing HSP47 siRNA alone or in combination with immunofluorescent dye (Dy647) were composed of similar compounds, as described previously.10 To track in vivo distribution of VA-lip HSP47, we intravenously injected Dy647-labeled VA-lip HSP47 (VA-lip Dy647) at a dose of 4.5 mg/kg at 3 times every 2 hours to mice with established bleomycin-induced localized scleroderma; we harvested skin samples 1 hour after the last injection. VA-lip HSP47 at a dose of 4.5 mg/kg were injected 3 times per week after BMT. In some experiments, VA-lip containing scramble siRNA with the same nucleotide composition as HSP47 siRNA were used as a control.
Cell culture
Skin fibroblasts were prepared from naïve BALB/c mice, as described previously.16 In brief, skin flap was digested in Dulbecco’s modified Eagle medium containing 5 mg/mL of type IV collagenase (Sigma-Aldrich Japan, Tokyo, Japan) for 1 hour, and subsequently cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum for 2 to 3 days in a 96-well plate at 37°C. After starvation for 12 hours in fetal calf serum–free medium, 5 × 103 of primary harvested skin fibroblasts or NIH/3T3 cells were stimulated with recombinant human transforming growth factor β1 (rhTGF-β1, R&D Systems, Minneapolis, MN) at a concentration of 5 ng/mL in the presence or absence of 50 nM of VA-lip HSP47 or VA-lip containing scramble siRNA. At 12 hours later, cells were collected and total RNA was extracted using ISOGEN II (Nippon Gene, Tokyo, Japan).
qPCR
We synthesized complementary DNA using ReverTra Ace quantitative polymerase chain reaction (qPCR) RT Master Mix with genomic DNA remover (Toyobo, Osaka, Japan), specific primers/probe sets. qPCR was performed on Applied Biosystems Step One Plus using TaqMan Universal PCR master mix and specific primers and probes purchased from Sigma-Aldrich Japan. The relative amount of each messenger RNA was calculated by the ΔCt method with Gapdh as the reference gene. Sequences of primers and probes are as follows: mouse Hsp47(Serpinh1): forward primer 5′-CTGCTTGTGAACGCCATGTTC-3′, reverse primer 5′-TCACCATGAAGCCACGGTTG-3′, probe 5′-6-AGCCACACTGGGATGAGAAGTTTCACCA-TAMRA-3′; mouse Gapdh: forward primer 5′-TGTCAAGCTCATTTCCTGGTATGA-3′, reverse primer 5′-TTGGGATAGGGCCTCTCTCTTGC-3′, probe 5′-6-TGGTGGACCTCATGGCCTACATGGCC-TAMRA-3′.
Collagen assay
Skin samples were harvested by a 5-mm punch biopsy technique using DermaPunch (Nipro, Osaka, Japan) and digested in 0.5M acetic acid and 0.1 mg/mL of pepsin solution for 48 hours. The amount of collagen was measured with a Sircol Collagen Assay kit (Biocolor, Carrickfergus, Northern Ireland, United Kingdom) and GloMax-Multi Luminescence System (Promega, Tokyo, Japan) according to manufacturers’ instructions. The collagen amount in each sample was divided by 19.6 to calculate collagen amount per 1-mm2 skin area.
Immunofluorescent analysis
After antigen retrieval with Target Retrieval Solution, Citrate pH6 (DAKO, Tokyo, Japan), paraffin-embedded sections were blocked with goat serum in Block Ace (DS Pharma Biomedical, Osaka, Japan), followed by incubation with primary Abs, such as anti–retinol-binding protein 1 (RBP1; Abcam, Cambridge, United Kingdom), anti-HSP47 (Abcam), anti-TGF-β (Abcam), and biotinylated anti–α-smooth muscle actin (α-SMA; Abcam) Abs diluted to 1:200 for overnight at 4°C. Freshly frozen sections were fixed with 4% paraformaldehyde and incubated with primary Abs, such as anti-HSP47 and biotinylated anti-F4/80 (Biolegend, San Diego, CA) diluted to 1:200 for 2 hours at room temperature. Primary Abs were then visualized with anti-rabbit immunoglobulin G or anti-goat immunoglobulin G conjugated to Alexa Fluor 488, Alexa Fluor 555 or Alexa Fluor 647, or streptavidin conjugated to Alexa Fluor 555. To detect Dy647-labeled VA-lip HSP47, frozen skin sections were prepared after fixation with 4% paraformaldehyde overnight and with 30% sucrose for the following 24 hours. Nuclear staining was done with 1 μg/mL of 4’,6-diamidino-2-phenylindole (DAPI; Dojindo Laboratories, Kumamoto, Japan). Images of tissue sections were acquired at room temperature using a FV-1000 (Olympus, Tokyo, Japan) or BZ-X700 (Keyence) with a 20×/0.75 NA or a 40×/1.30 NA objective lens. Data were analyzed with Olympus FluoView software (Olympus) and Keyence BZ-X Analyzer software. Signals of each channel of immunofluorescent images are shown in supplemental Figure 1, available on the Blood Web site. Quantification of fluorescence on stained sections was performed with Aperio Image Scope software (Aperio Technologies, Vista, CA), automatically counting the number of positive pixels in the dermal area, as shown previously.17
Statistical analysis
We used the Mann-Whitney U test to compare data. Survival probabilities were plotted using the Kaplan-Meier method, and the log-rank test was applied to compare survival curves. We performed analyses using Prism software version 6 (GraphPad, La Jolla, CA).
Results
Accumulation of HSP47+ myofibroblasts in the sclerodermatous skin lesion of chronic GVHD
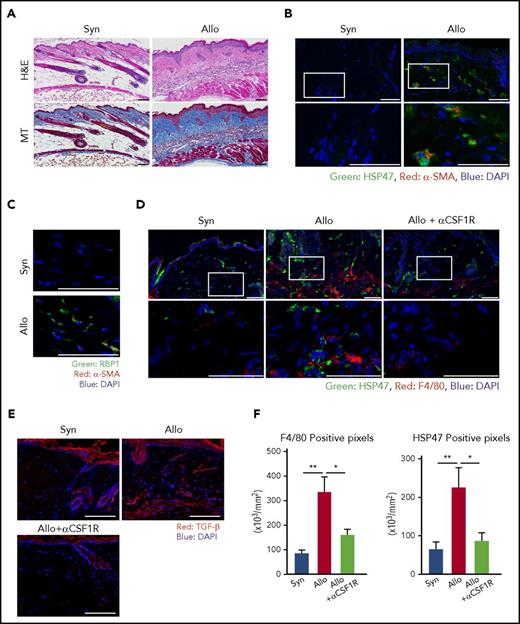
Myofibroblasts secrete excessive amounts of collagen and are responsible for fibrosis in various disorders, such as liver cirrhosis, systemic sclerosis, and chronic GVHD.18,19 We initially examined if myofibroblasts could accumulate in the sclerodermatous lesion of chronic GVHD in a well-established murine model of cutaneous chronic GVHD.12 BALB/c mice were conditioned with 6 Gy of TBI and intravenously injected with 8 × 106 BM cells plus 2.5 × 107 splenocytes from allogeneic B10.D2 or syngeneic BALB/c donors. H&E and MT staining of the skin samples harvested on day +42 after BMT demonstrated classical pathological changes of chronic GVHD, such as reduced hair follicles and fat layer with massive fibrosis of the dermis (Figure 1A). Immunofluorescent staining showed an accumulation of α-SMA+ myofibroblasts in the dermis of allogeneic animals, but not in syngeneic controls (Figure 1B; supplemental Figure 1A). These myofibroblasts co-expressed HSP47, a collagen-specific molecular chaperone,9 and RBP1, a cellular retinol-binding protein (Figure 1B-C; supplemental Figure 1A-B and 2A). 3D images reconstructed from multichannel z-stack images of triple immunofluorescent staining of RBP1, HSP47, and α-SMA further confirmed co-expression of these 3 molecules, suggesting that these cells could be targeted by VA-lip HSP47 (supplemental Figure 2B).
HSP47+myofibroblasts are accumulated in the fibrotic skin lesion of chronic GVHD. BALB/c mice were transplanted with 8 × 106 BM cells plus 2.5 × 107 splenocytes from allogeneic B10.D2 or syngeneic BALB/c donors on day 0, following 6 Gy of TBI. Skin samples were harvested on day +42 after BMT for histological analyses. (A) H&E and MT images. (B) Immunofluorescent images for HSP47 (green) and α-SMA (red) with DAPI (blue) counterstaining. The area in the white rectangles is magnified and is shown below the original images. (C) Immunofluorescent images for RBP1 (green) and α-SMA (red) with DAPI (blue) counterstaining. (D-F) A group of mice were intraperitoneally injected with 0.5 mg/body of αCSF1R 3 times per week after BMT, and skin samples were harvested on day +42. (D) Immunofluorescent images for HSP47 (green) and F4/80 (red) with DAPI (blue) counterstaining. The area in the white rectangles is magnified and is shown below the original images. (E) Immunofluorescent images for TGF-β (red) with DAPI (blue) counterstaining. (F) Positive pixels of F4/80 (left) and HSP47 (right) on skin samples from allogeneic and syngeneic controls and αCSF1R-treated allogeneic mice (n = 7-10/group). Data from 2 independent experiments were combined and are shown as means ± standard error of the mean (SEM). Magnification, ×20. Scale bar, 50 μm. *P < .05; **P < .01. Allo, allogeneic; Syn, syngeneic.
HSP47+myofibroblasts are accumulated in the fibrotic skin lesion of chronic GVHD. BALB/c mice were transplanted with 8 × 106 BM cells plus 2.5 × 107 splenocytes from allogeneic B10.D2 or syngeneic BALB/c donors on day 0, following 6 Gy of TBI. Skin samples were harvested on day +42 after BMT for histological analyses. (A) H&E and MT images. (B) Immunofluorescent images for HSP47 (green) and α-SMA (red) with DAPI (blue) counterstaining. The area in the white rectangles is magnified and is shown below the original images. (C) Immunofluorescent images for RBP1 (green) and α-SMA (red) with DAPI (blue) counterstaining. (D-F) A group of mice were intraperitoneally injected with 0.5 mg/body of αCSF1R 3 times per week after BMT, and skin samples were harvested on day +42. (D) Immunofluorescent images for HSP47 (green) and F4/80 (red) with DAPI (blue) counterstaining. The area in the white rectangles is magnified and is shown below the original images. (E) Immunofluorescent images for TGF-β (red) with DAPI (blue) counterstaining. (F) Positive pixels of F4/80 (left) and HSP47 (right) on skin samples from allogeneic and syngeneic controls and αCSF1R-treated allogeneic mice (n = 7-10/group). Data from 2 independent experiments were combined and are shown as means ± standard error of the mean (SEM). Magnification, ×20. Scale bar, 50 μm. *P < .05; **P < .01. Allo, allogeneic; Syn, syngeneic.
CSF1R-dependent macrophages play a critical role in the accumulation of HSP47+ myofibroblasts in the chronic GVHD skin
Immunofluorescence studies demonstrated accumulation of F4/80+ macrophages juxtaposed to HSP47+ myofibroblasts in the dermis of chronic GVHD skin (Figure 1D; supplemental Figure 1C), in association with abundant production of a profibrotic cytokine, TGF-β (Figure 1E; supplemental Figure 1D). We evaluated the role of CSF1R signaling on the accumulation of macrophages and myofibroblasts in the skin lesion. Recipient mice were intraperitoneally injected with 0.5 mg/body of αCSF1R mAbs at 3 times per week after BMT, resulting in marked reduction of F4/80+ macrophages in the skin of chronic GVHD (Figure 1D,F; supplemental Figure 1C). Depletion of macrophages abrogated the production of TGF-β and accumulation of HSP47+ myofibroblasts (Figure 1D-F; supplemental Figure 1C-D), indicating the critical role of CSF1R-dependent macrophages in the accumulation of HSP47+ myofibroblasts in the fibrotic skin lesion of chronic GVHD.
VA-lip HSP47 inhibit HSP47 expression in TGF-β–stimulated fibroblasts in vitro
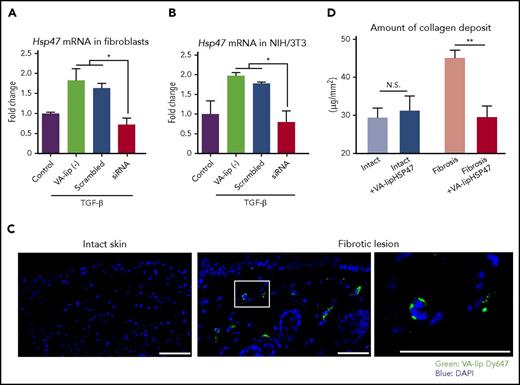
Given the accumulation of HSP47+ myofibroblasts in the chronic GVHD skin, we hypothesized that VA-lip HSP47 could ameliorate skin fibrosis in chronic GVHD by knocking down Hsp47 in myofibroblasts. To test this hypothesis, we initially evaluated if VA-lip HSP47 could knock down Hsp47 expression in myofibroblasts in vitro. TGF-β is a potent inducer of myofibroblastic differentiation of fibroblasts.20 Stimulation with rhTGF-β for 12 hours significantly increased expression of Hsp47 in primary skin fibroblasts isolated from naïve mice. Addition of VA-lip HSP47 into the culture completely abrogated TGF-β–mediated Hsp47 upregulation, whereas VA-lip containing scramble siRNA did not affect Hsp47 expression in myofibroblasts, confirming the sequence-specific effects of VA-lip HSP47 (Figure 2A). Similar results were obtained in experiments using murine fibroblast cell line NIH/3T3 (Figure 2B).
VA-lip HSP47 knock down HSP47 expression in myofibroblasts and ameliorate bleomycin-induced skin fibrosis. (A-B) Primary skin fibroblasts isolated from naïve BALB/c mice (A) and NIH/3T3 (B) were stimulated with 5 ng/mL of rhTGF-β in the presence or absence of VA-lip containing scramble siRNA or VA-lip HSP47 for 12 hours. Hsp47 messenger RNA levels relative to Gapdh were determined using qPCR, and fold differences relative to those of unstimulated control cells are shown (n = 3/group). Data from one of 2 independent experiments with similar results are shown. (C-D) B6 mice were subcutaneously injected with bleomycin daily for 21 days. (C) On the next day of the last bleomycin treatment, mice were intravenously injected with VA-lip Dy647 at 3 times every 2 hours, and frozen sections of skin were prepared at 3 hours after the last injection. Fluorescence of VA-lip Dy647 (green) was visualized with DAPI (blue) counterstaining. The area in the white rectangle is magnified and is shown on the right side of the original image. Magnification, ×20 or ×40. Scale bar, 50 μm. (D) A group of mice were treated with VA-lip HSP47 at 3 times per week from day 1 of bleomycin treatment. The amount of collagen deposit in the fibrotic lesion or the intact skin from VA-lip HSP47–treated (n = 9) or untreated mice (n = 17) from 2 independent experiments was combined and is shown as means ± SEM. *P < .05; **P < .01. N.S., not significant.
VA-lip HSP47 knock down HSP47 expression in myofibroblasts and ameliorate bleomycin-induced skin fibrosis. (A-B) Primary skin fibroblasts isolated from naïve BALB/c mice (A) and NIH/3T3 (B) were stimulated with 5 ng/mL of rhTGF-β in the presence or absence of VA-lip containing scramble siRNA or VA-lip HSP47 for 12 hours. Hsp47 messenger RNA levels relative to Gapdh were determined using qPCR, and fold differences relative to those of unstimulated control cells are shown (n = 3/group). Data from one of 2 independent experiments with similar results are shown. (C-D) B6 mice were subcutaneously injected with bleomycin daily for 21 days. (C) On the next day of the last bleomycin treatment, mice were intravenously injected with VA-lip Dy647 at 3 times every 2 hours, and frozen sections of skin were prepared at 3 hours after the last injection. Fluorescence of VA-lip Dy647 (green) was visualized with DAPI (blue) counterstaining. The area in the white rectangle is magnified and is shown on the right side of the original image. Magnification, ×20 or ×40. Scale bar, 50 μm. (D) A group of mice were treated with VA-lip HSP47 at 3 times per week from day 1 of bleomycin treatment. The amount of collagen deposit in the fibrotic lesion or the intact skin from VA-lip HSP47–treated (n = 9) or untreated mice (n = 17) from 2 independent experiments was combined and is shown as means ± SEM. *P < .05; **P < .01. N.S., not significant.
VA-lip HSP47 specifically target the fibrotic skin lesion
To test if VA-lip HSP47 could specifically target myofibroblasts in the fibrotic skin lesion, VA-lip Dy647 were intravenously injected to the mice with established localized fibrotic skin lesion induced by subcutaneous injection of bleomycin. Dy647-labeled particles were specifically distributed in the fibrotic lesion of the skin but not in healthy skin (Figure 2C; supplemental Figure 1E). When mice were treated with VA-lip HSP47 at 3 times per week for 3 weeks, VA-lip HSP47 normalized the amount of collagen deposit in bleomycin-treated skin but did not modify the amount of collagen deposit in the intact skin of the same mice (Figure 2D).
VA-lip HSP47 ameliorate fibrosis of the skin and salivary glands in chronic GVHD
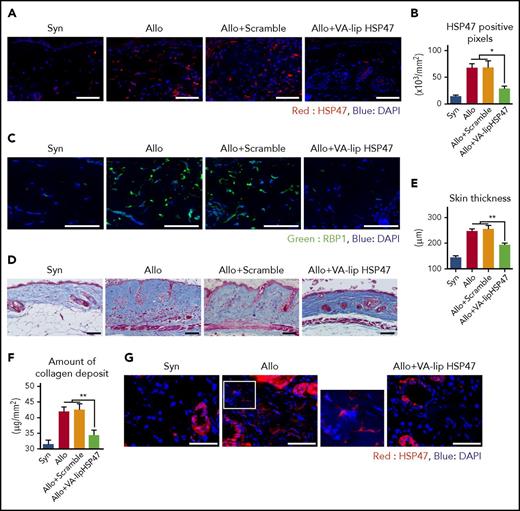
We then assessed the antifibrotic effects of VA-lip HSP47 in chronic GVHD. VA-lip HSP47 were intravenously injected at a dose of 4.5 mg/kg at 3 times per week from day +2 after BMT. Immunofluorescent studies on day +42 showed that VA-lip HSP47, but not VA-lip containing scramble siRNA, knocked down both HSP47 and RBP1 in the skin after allogeneic BMT (Figure 3A-C; supplemental Figure 1F-G), suggesting that VA-lip HSP47 targeted HSP47+ and RBP1+ myofibroblasts. MT staining showed that VA-lip HSP47 ameliorated skin thickening and collagen deposition (Figure 3D-E). Sircol Collagen Assay confirmed that VA-lip HSP47 significantly reduced collagen load in the skin of allogeneic recipients (Figure 3F). VA-lip containing scramble siRNA did not ameliorate cutaneous fibrosis, ruling out possible role of vitamin A, a constituent of VA-lip, in the prophylactic effects of VA-lip HSP47 against chronic GVHD (Figure 3A-F; supplemental Figure 1F-G).
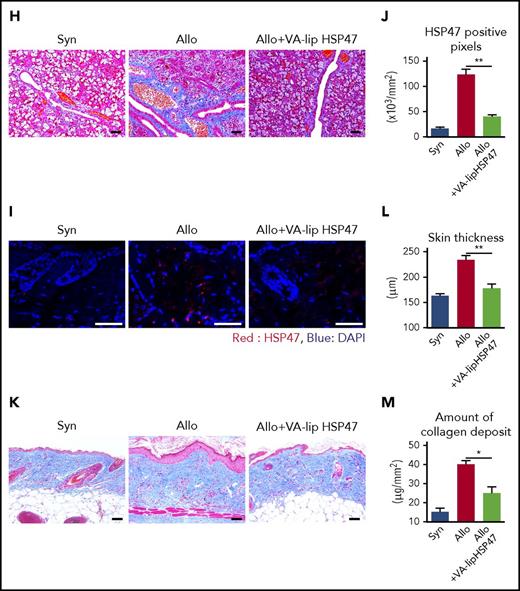
VA-lip HSP47 ameliorate fibrosis of the skin and salivary glands in chronic GVHD. (A-F) Mice were transplanted as in Figure 1. A group of allogeneic recipients were treated with VA-lip HSP47 or VA-lip containing scramble siRNA at 3 times per week from day +2 to day +41, and skin samples were harvested on day +42 after BMT. (A) Immunofluorescent images for HSP47 (red) with DAPI (blue) counterstaining. Magnification, ×40. Scale bar, 50 µm. (B) Numbers of positive pixels for HSP47 in syngeneic (n = 9), allogeneic controls (n = 13), and allogeneic recipients treated with VA-lip containing scramble siRNA (n = 12) or VA-lip HSP47 (n = 13) from 2 of 3 independent experiments were combined and shown as means ± SEM. (C) Immunofluorescent images for RBP1 (green) with DAPI (blue) counterstaining. Magnification, ×40. Scale bar, 50 µm. (D) Representative images of MT staining. Magnification, ×20. Scale bar, 50 µm. Skin thickness (E) and amount of collagen deposit (F) in syngeneic (n = 19), allogeneic controls (n = 28), and allogeneic recipients treated with VA-lip containing scramble siRNA (n = 15) or VA-lip HSP47 (n = 21) from 5 independent experiments were combined and shown as means ± SEM. (G-H) Submandibular glands were harvested on day +42 after BMT. (G) Immunofluorescent images for HSP47 (red) with DAPI (blue) counterstaining. Magnification, ×40. Scale bar, 50 µm. The area in the white rectangle is magnified and is shown on the right side of the original image. (H) Representative images of MT staining. Magnification, ×20. Scale bar, 50 µm. (I-M) Lethally irradiated B6 mice were transplanted with G-CSF–mobilized splenocytes from B6 (Syn, n = 10) or BALB/c (Allo, n = 21) donors. Allogeneic recipients were treated with VA-lip HSP47 (n = 7) or diluent (n = 14) at 3 times per week from day +2 to day +41, and skin samples were harvested on day +42 after BMT. (I) Immunofluorescent images for HSP47 (red) with DAPI (blue) counterstaining. Magnification, ×40. Scale bar, 50 µm. (J) Numbers of positive pixels for HSP47 in syngeneic, allogeneic controls, and allogeneic recipients treated with VA-lip HSP47. (K) Representative images of MT staining. Magnification, ×20. Scale bar, 50 µm. Skin thickness (L) and amount of collagen deposit (M) in syngeneic, allogeneic controls, and allogeneic recipients treated with VA-lip HSP47. Data from 2 independent experiments were combined and are shown as means ± SEM. *P < .05; **P < .01.
VA-lip HSP47 ameliorate fibrosis of the skin and salivary glands in chronic GVHD. (A-F) Mice were transplanted as in Figure 1. A group of allogeneic recipients were treated with VA-lip HSP47 or VA-lip containing scramble siRNA at 3 times per week from day +2 to day +41, and skin samples were harvested on day +42 after BMT. (A) Immunofluorescent images for HSP47 (red) with DAPI (blue) counterstaining. Magnification, ×40. Scale bar, 50 µm. (B) Numbers of positive pixels for HSP47 in syngeneic (n = 9), allogeneic controls (n = 13), and allogeneic recipients treated with VA-lip containing scramble siRNA (n = 12) or VA-lip HSP47 (n = 13) from 2 of 3 independent experiments were combined and shown as means ± SEM. (C) Immunofluorescent images for RBP1 (green) with DAPI (blue) counterstaining. Magnification, ×40. Scale bar, 50 µm. (D) Representative images of MT staining. Magnification, ×20. Scale bar, 50 µm. Skin thickness (E) and amount of collagen deposit (F) in syngeneic (n = 19), allogeneic controls (n = 28), and allogeneic recipients treated with VA-lip containing scramble siRNA (n = 15) or VA-lip HSP47 (n = 21) from 5 independent experiments were combined and shown as means ± SEM. (G-H) Submandibular glands were harvested on day +42 after BMT. (G) Immunofluorescent images for HSP47 (red) with DAPI (blue) counterstaining. Magnification, ×40. Scale bar, 50 µm. The area in the white rectangle is magnified and is shown on the right side of the original image. (H) Representative images of MT staining. Magnification, ×20. Scale bar, 50 µm. (I-M) Lethally irradiated B6 mice were transplanted with G-CSF–mobilized splenocytes from B6 (Syn, n = 10) or BALB/c (Allo, n = 21) donors. Allogeneic recipients were treated with VA-lip HSP47 (n = 7) or diluent (n = 14) at 3 times per week from day +2 to day +41, and skin samples were harvested on day +42 after BMT. (I) Immunofluorescent images for HSP47 (red) with DAPI (blue) counterstaining. Magnification, ×40. Scale bar, 50 µm. (J) Numbers of positive pixels for HSP47 in syngeneic, allogeneic controls, and allogeneic recipients treated with VA-lip HSP47. (K) Representative images of MT staining. Magnification, ×20. Scale bar, 50 µm. Skin thickness (L) and amount of collagen deposit (M) in syngeneic, allogeneic controls, and allogeneic recipients treated with VA-lip HSP47. Data from 2 independent experiments were combined and are shown as means ± SEM. *P < .05; **P < .01.
Chronic GVHD is a complex disorder that affects multiple organs. We next tested if VA-lip HSP47 could ameliorate chronic GVHD–induced fibrosis in the salivary gland, another representative target organ of chronic GVHD.3 Immunofluorescent and histological studies of the submandibular glands harvested on day +42 after allogeneic SCT demonstrated significant fibrosis and accumulation of HSP47+ myofibroblasts in the interstitial space of the salivary glands (Figure 3G-H; supplemental Figure 1H). VA-lip HSP47 abrogated accumulation of HSP47+ myofibroblasts and collagen deposition in the salivary glands (Figure 3G-H; supplemental Figure 1H). Antifibrotic effects of VA-lip HSP47 was not strain dependent, as VA-lip HSP47 abrogated accumulation of HSP47+ myofibroblasts and ameliorated skin fibrosis in another mouse model of cutaneous chronic GVHD in which lethally irradiated B6 mice were transplanted with G-CSF–mobilized splenocytes from BALB/c donors (Figure 3I-M; supplemental Figure 1I).
Effects of VA-lip HSP47 were limited to fibrotic lesions. Administration of VA-lip HSP47 did not affect mortality and reconstitution of CD4+CD8+ double-positive thymocytes and CD4+ and CD8+ T cells in the spleen after BMT (supplemental Figure 3A-C). Administration of VA-lip HSP47 did not impair donor cell engraftment (supplemental Figure 3D). Taken together, VA-lip HSP47 suppressed pathological skin fibrosis without modulating systemic GVHD, donor engraftment, and immune reconstitution.
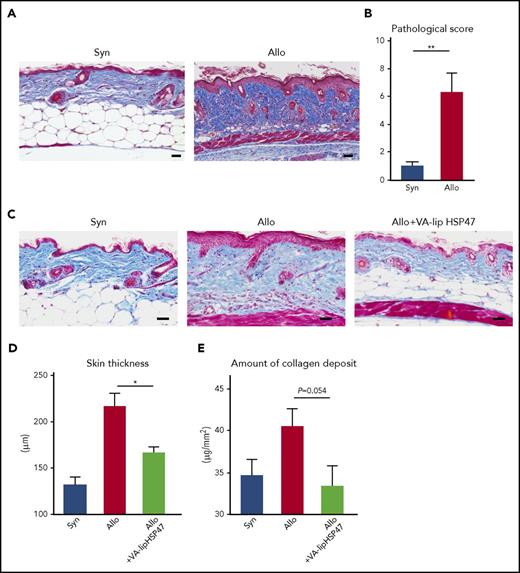
Finally, we tested if VA-lip HSP47 could promote resolution of established skin fibrosis in chronic GVHD. In this chronic GVHD model, pathological GVHD including skin fibrosis can be detected by day +21 (Figure 4A-B). VA-lip HSP47 were injected at 3 times per week to allogeneic recipient mice from day +21 to day +41 after BMT. Skin thickening and amount of collagen deposit were assessed on day +42 after BMT. VA-lip HSP47 significantly mitigated skin thickening and collagen deposition (Figure 4C-E). Altogether, these results indicate the prophylactic and therapeutic roles of VA-lip HSP47 against fibrotic chronic GVHD.
VA-lip HSP47 resolve established skin fibrosis in chronic GVHD. Mice were transplanted as in Figure 1. VA-lip HSP47 were intravenously injected 3 times per week from day +21 to day +41 into allogeneic recipients. Representative images of MT staining (A) and skin pathological GVHD scores (B) on day +21. (C-E) The skin samples were harvested on day +42 after BMT. (C) Representative images of MT staining. Skin thickness (D) and amount of collagen deposit (E) on day +42 in the skin of syngeneic (n = 7), allogeneic (n = 14), and allogeneic recipients treated with VA-li pHSP47 (n = 11) from 2 independent experiments were combined and are shown as means ± SEM. Scale bar, 50 µm. *P < .05; **P < .01.
VA-lip HSP47 resolve established skin fibrosis in chronic GVHD. Mice were transplanted as in Figure 1. VA-lip HSP47 were intravenously injected 3 times per week from day +21 to day +41 into allogeneic recipients. Representative images of MT staining (A) and skin pathological GVHD scores (B) on day +21. (C-E) The skin samples were harvested on day +42 after BMT. (C) Representative images of MT staining. Skin thickness (D) and amount of collagen deposit (E) on day +42 in the skin of syngeneic (n = 7), allogeneic (n = 14), and allogeneic recipients treated with VA-li pHSP47 (n = 11) from 2 independent experiments were combined and are shown as means ± SEM. Scale bar, 50 µm. *P < .05; **P < .01.
Discussion
Chronic GVHD is characterized by multiorgan fibrosis and dysfunction.3 Sclerodermatous chronic GVHD is one of the severe manifestations of chronic GVHD, with an incidence of 15.5% in patients with chronic GVHD.21 Fibrotic injury is characterized by excessive accumulation of extracellular matrix such as collagen, eventually leading to organ malfunction.18 After the inflammatory phase subsides, an initiating event of fibrotic diseases is the activation of both recruiting and resident macrophages. They secrete numerous profibrotic cytokines such as TGF-β, which subsequently induce differentiation of fibroblasts into myofibroblasts, the key mediators of fibrotic tissue remodeling.7,18-20 A macrophage depletion study by injection of αCSF1R-blocking Abs showed that production of TGF-β and accumulation of myofibroblasts in the skin depended on macrophages (Figure 1D-F). Our results are consistent with a recent study demonstrating that depletion of macrophages by αCSF1R mAbs markedly reduces GVHD pathology.17 Taken together, targeting CSF1 signaling may be a promising strategy to modulate skin chronic GVHD by inhibiting production of fibrosis-prone cytokines.17,22,23 However, depletion of recipient macrophages using αCSF1R mAbs before BMT exaggerates experimental acute GVHD,14,24 indicating that the timing of macrophage depletion is critical for the outcome of SCT.
In pathological conditions, myofibroblasts continue to present in the tissue, leading to excessive fibrosis.7,18 We demonstrated that myofibroblasts were accumulated in the fibrotic skin and salivary glands in mice with chronic GVHD. A previous study showed an increase of myofibroblasts in the skin biopsy samples from patients with chronic GVHD.25 HSP47 plays a role in the maturation of procollagen to collagen.9 Although overexpression of HSP47 has been shown in various fibrotic diseases,26-29 a few studies have investigated HSP47 expression in transplantation medicine: increased HSP47 expression in the kidney in renal allograft failure30 and in the lacrimal glands in patients with chronic GVHD.31 In this study, we demonstrated increased expression of HSP47 in myofibroblasts of the skin and salivary glands in experimental chronic GVHD. HSP47 is induced by TGF-β,9,32 which is a key cytokine in fibrosis in chronic GVHD, suggesting a critical role of HSP47 in this manifestation. Thus, we have uncovered a critical cellular and molecular cascade of fibrosis in chronic GVHD, including (1) macrophage production of TGF-β, (2) TGF-β–mediated activation of fibroblasts to differentiate into myofibroblasts to express HSP47, and (3) HSP47-mediated myofibroblast production of collagen.
VA-lip HSP47 are conjugated to RBPs, are uptaken by cells expressing RBP receptors, and deliver siRNA against HSP47.10 We have shown that VA-lip HSP47 are uptaken by hepatic stellate cells, which differentiate into myofibroblasts and myofibroblasts in the pancreas and modulate liver and pancreas fibrosis, respectively.10,11 In our study, we found that myofibroblasts in chronic skin GVHD express HSP47 and RBP1, suggesting that these cells can be targets of VA-lip HSP47. As expected, VA-lip HSP47 delivered HSP47 siRNA to myofibroblasts in the dermis of the fibrotic lesion and knocked down their HSP47. Administration of VA-lip HSP47 prevented and reversed collagen deposition in the skin and salivary glands of chronic GVHD.
Myofibroblasts are not present in healthy tissues, but tissue-resident fibroblasts produce collagen at a lower rate compared with myofibroblasts.33 However, it remains to be elucidated if their expression levels differ between fibroblasts in healthy tissue and myofibroblasts in fibrotic lesions. Our study demonstrated that intravenously injected VA-lip HSP47 were selectively distributed to the skin fibrotic lesions, suggesting that fibroblasts residing in healthy skin might express fewer vitamin A receptors than pathogenic myofibroblasts. Furthermore, VA-lip HSP47 suppress fibrosis without affecting healthy skin. These results suggest that only myofibroblasts in fibrotic lesions express sufficient levels of both vitamin A receptors and HSP47 to be targeted by VA-lip HSP47. It is also important to evaluate if VA-lip HSP47 could have any effects on vitamin A receptor–expressing cells other than myofibroblasts. Toxicity profiles in ongoing clinical studies are yet to be analyzed. Furthermore, more extensive studies of on-and-off target immune effects are required. In this regard, ex vivo studies using human skin explants and further evaluation of the immunological effects of VA-lip HSP47 may help to understand the mechanism of antifibrotic effects in chronic GVHD and healthy tissues more precisely.34
Recently, antifibrotic agents have been developed to treat systemic sclerosis, pulmonary fibrosis, and liver cirrhosis. Pirfenidone, an US Food and Drug Administration–approved antifibrotic agent used to treat idiopathic lung fibrosis,35 ameliorates experimental chronic GVHD in the lung and skin via inhibition of macrophage infiltration and TGF-β production.23 MiR-29b also suppresses the expression of HSP47 in fibrosis.36 Fresolimumab, an anti–TGF-β mAb, has been shown to reduce HSP47 expression and ameliorate fibrosis in the skin of patients with systemic sclerosis.37 Mesoporous silica nanoparticles containing HSP47 siRNA also have been shown to ameliorate skin fibrosis, although their selectivity of delivery of HSP47 siRNA was not clear.38 In contrast, selectivity of the delivery of VA-lip HSP47 to the affected lesions has been confirmed in our current study and previous studies from other groups.10 VA-lip HSP47 are now being used in clinical trials of human liver cirrhosis (https://clinicaltrials.gov/ct2/results?cond=&term=ND-L02). Because VA-lip HSP47 do not suppress donor T-cell responses, VA-lip HSP47 represent a novel adjunct to standard immunosuppressive GVHD prophylaxis and treatment in patients with chronic fibrotic GVHD. In conclusion, VA-lip HSP47 are a new and potentially promising antifibrotic therapy for chronic GVHD.
Presented in abstract form at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 5 December 2016.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by JSPS KAKENHI grants 17H04206 and 25293217 (T.T.) and grant 26461438 (D.H.), Promotion and Standardization of the Tenure-Track System (D.H.), and the Center of Innovation Program from the Japan Science and Technology Agency (T.T.).
Authorship
Contribution: T.T., D.H., Y.N., and M.O. developed the conceptual framework of the study, designed the experiments, conducted studies, analyzed the data, and wrote the paper; T.Y., H.O., E.H., S.T., and M.M. conducted the experiments and analyzed the data; and K.M. supervised the experiments.
Conflict-of-interest disclosure: M.M. and K.M. are employees at Nitto Denko Corporation. The remaining authors declare no competing financial interests.
Correspondence: Takanori Teshima, Department of Hematology, Faculty of Medicine, Hokkaido University, N15 W7, Kita-Ku, Sapporo 060-8638, Japan; e-mail: teshima@med.hokudai.ac.jp.
REFERENCES
Author notes
T.Y., H.O., and D.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal