Key Points
BV and Nivo were well-tolerated in patients with R/R HL, with less than 10% of patients treated with systemic steroids for immune-related AEs.
The complete response rate was 61% (82% objective response rate), and patients were able to undergo stem cell transplant without adverse impact.
Abstract
In this phase 1/2 study, brentuximab vedotin (BV) and nivolumab (Nivo) administered in combination were evaluated as initial salvage therapy in patients with relapsed or refractory (R/R) classical Hodgkin lymphoma (HL). Patients received up to 4 cycles of combination treatment, with BV administered on day 1 and Nivo on day 8 of the first cycle. For cycles 2 to 4, BV and Nivo were both administered on day 1. After study treatment, responses were evaluated by investigators per the 2014 Lugano classification, and patients could proceed to autologous stem cell transplantation (ASCT). Sixty-two patients were enrolled; the complete response rate among all treated patients (n = 61) was 61%, with an objective response rate of 82%. Before ASCT, adverse events (AEs) occurred in 98% of patients, mostly grades 1 and 2. Infusion-related reactions (IRRs) occurred in 44% of patients overall, with 41% of patients experiencing an IRR during at least 1 infusion of BV. Five patients (8%) were treated with systemic steroids for immune-related AEs. A reduction of peripheral T-cell subsets including regulatory T cells was observed after the first dose of BV, and reduced serum levels of thymus- and activation-regulated chemokine concurrent with an increase in proinflammatory cytokines and chemokines were seen after the first BV plus Nivo infusions. The combination of BV plus Nivo was an active and well-tolerated first salvage regimen, potentially providing patients with R/R HL an alternative to traditional chemotherapy. This trial was registered at www.clinicaltrials.gov as #NCT02572167.
Introduction
Up to 70% to 90% of patients with classical Hodgkin lymphoma (HL) treated with standard chemotherapy or chemoradiotherapy experience durable remissions, although ∼10% to 30% will become refractory to initial therapy or will relapse.1-5 Standard-of-care treatment of relapsed or refractory HL (R/R HL) is multiagent salvage chemotherapy followed by autologous stem cell transplantation (ASCT) in patients who are chemosensitive. Approximately 70% to 90% of patients will have an objective response to platinum- or gemcitabine-based salvage combination chemotherapy, and 50% to 75% of patients achieve a complete remission as assessed by positron emission tomography (PET) scan.6-13 Durable remission is attained in approximately half of patients who undergo ASCT; patients who have a negative result on PET scan before ASCT most likely will retain long-term disease control.14-21
Brentuximab vedotin (BV) and nivolumab (Nivo) are well-tolerated and effective treatments for R/R HL that are not traditional chemotherapies. In addition to direct cytotoxicity achieved by delivery of a potent anti-tubulin payload to CD30-positive Reed-Sternberg (RS) cells, BV may activate the innate immune system and initiate an antitumor immune response through the induction of immunogenic cell death via endoplasmic reticulum stress.22,23 As a single agent, BV produces an objective response rate (ORR) of 72% and a complete response (CR) rate of 33% in patients with R/R HL.24 Nivo is an inhibitor of the programmed death-1 (PD-1) receptor, and tumor cells expressing the PD-1 ligands, PD-L1 and PD-L2, can exploit the PD-1 pathway to evade an antitumor immune response.25 The PD-1 pathway appears critical in the pathogenesis of HL because chromosome 9p24.1 alterations in RS cells result in overexpression of PD-L1 and PD-L2,26,27 and PD-L1 is expressed on immune cells in the HL tumor microenvironment.28,29 In patients with R/R HL, Nivo treatment results in a 73% ORR and a 28% CR rate (by investigator assessment).30
When used as first salvage therapy for patients with R/R HL, BV yielded a CR rate of 27% to 35%, with ∼90% of patients ultimately proceeding to ASCT after BV alone or BV followed by ifosfamide, carboplatin, and etoposide (ICE), or other multiagent chemotherapy regimens.31,32 With the implementation of such an approach, 27% to 48% of patients were able to proceed directly to ASCT after BV alone, and avoid multiagent salvage chemotherapy, which can be associated with negative downstream health consequences and morbidity.33,34 However, in light of the important prognostic impact of pre-ASCT metabolic CR, further improvement in the CR rate is needed. Given the high single-agent response rates observed with each agent, we hypothesized that this combination could be an effective treatment regimen for R/R HL that spares patients traditional chemotherapy before ASCT.
Patients and methods
We conducted a phase 1/2, open-label, multicenter study of BV in combination with Nivo (ClinicalTrials.gov: NCT02572167) in patients with refractory HL (defined as not achieving a CR to frontline therapy or progression within 3 months of CR), or HL that had relapsed (defined as progression ≥3 months after CR to frontline therapy).
To be enrolled on this trial, ASCT-eligible patients ≥18 years old must have had biopsy-proven R/R disease after failure of standard frontline chemotherapy, with 18-F fluorodeoxyglucose (FDG)-PET avid and bidimensional measurable disease of at least 1.5 cm, and an Eastern Cooperative Oncology Group (ECOG) performance score of 0 to 1. Patients were excluded if they had received prior salvage therapy for R/R HL (including salvage radiotherapy), prior BV or immuno-oncology therapy, prior autologous or allogeneic SCT, radiation therapy within 3 weeks, or chest radiation ≤12 weeks before first dose of study drug.
The study protocol and all amendments were approved by each site’s institutional review board and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines.
Study design and treatment
Patients received BV (1.8 mg/kg IV, 30-minute infusion) and Nivo (3.0 mg/kg IV, 60-minute infusion) in 3-week cycles for up to 12 weeks (4 cycles). During the first cycle, BV was administered on day 1 and Nivo on day 8. During cycles 2 to 4, BV and Nivo were administered on day 1, with Nivo given at least 30 minutes after BV. Additional salvage therapy before ASCT, ASCT, and any post-ASCT consolidative therapies was performed at the discretion of the treating physician according to institutional practices.
Study assessments
All adverse events (AEs) and serious AEs (SAEs), regardless of relationship to study drug, were recorded from study day 1 through 100 days after the last dose of Nivo, and included the ASCT period as applicable. A safety monitoring committee reviewed safety data and provided enrollment guidance in part 1 and expansion of enrollment in part 2.
A computed tomography scan was performed at cycle 2 to assess for progressive disease (PD). Response was assessed by both PET and computed tomography scans at the end of treatment (EOT), which occurred 30 to 37 days after the last dose of study drug. Responses were assessed by the investigator per the Revised Response Criteria for Malignant Lymphoma.35 The PET scan metabolic uptake was graded using the Deauville 5-point scale with a score of ≤3 considered a complete metabolic response (CMR).36,37
Statistical analysis
The primary efficacy end point was the CR rate after the completion of study treatment. Secondary end points included the ORR, progression-free survival (PFS) after ASCT, and duration of response (DOR). Additional efficacy end points included overall survival and PFS. The safety and all treated patient sets included patients who received at least 1 dose of BV or Nivo. The efficacy-evaluable set included all patients who received either drug and then subsequently underwent response assessment. CR rate was defined as the proportion of patients with CR at EOT, before ASCT or initiation of subsequent antitumor treatment. ORR was defined as the proportion of patients with CR or partial response (PR) at EOT.35 We calculated the 2-sided 95% exact binomial confidence interval (CI) for CR rate and ORR using the Clopper-Pearson method.38
We estimated DOR and PFS using the Kaplan-Meier method and calculated the associated 2-sided 95% CI using the log-log transformation method.39 DOR was defined as the time from first documentation of objective response to the first documentation of PD or to death from any cause. PFS was defined as the time from enrollment to the first documentation of PD or to death from any cause.39
We performed an analysis using the standard Medical Dictionary for Regulatory Activities (version 18.0) to identify peripheral neuropathy (PN) events. We then conducted a search of coded AE terms using a set of preferred terms representing potential immune-related AEs (IrAEs). Grade of severity was determined per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Biomarker assessment
Biomarkers in the peripheral blood were analyzed for immunophenotyping by flow cytometry, serum cytokine and chemokine quantification, T-cell receptor (TCR) clonality determined by receptor sequencing, and intracellular cytokine staining of ex vivo peptide–stimulated T cells. Multiple flow cytometry panels were performed by Q2 Solutions (Marietta, GA) on heparinized whole blood. Serum cytokines and chemokines were evaluated by enzyme-linked immunosorbent assay at Covance (Greenfield, IN), and using a Luminex platform at Myriad/Rules Based Medicine (Austin, TX). Peripheral blood mononuclear cells (PBMCs) were sent to Adaptive Biotechnologies (Seattle, WA) for TCRβ sequencing using the immunoSEQ platform. PBMCs were isolated from cell preparation tubes (BD Biosciences, San Jose, CA), frozen, and then analyzed in batches by Caprion Biosciences (Montreal, Quebec, Canada) using an intracellular cytokine-staining platform following peptide stimulation.
Results
Patients
Sixty-two patients with R/R HL were enrolled between 20 October 2015, and 23 November 2016, at 12 study sites in the United States. As of the 21 July 2017, data extract, all 62 patients had completed study treatment or discontinued from the study and had been observed through the safety reporting period. Among the 62 enrolled patients, 61 patients were treated with BV (median, 4 doses; range, 1-4 doses) and Nivo (median, 4 doses; range, 1-4 doses), and 58 patients completed all 4 cycles of treatment. Four patients discontinued study participation because of investigator decision (n = 1), PN AE (n = 1), and patient decision (n = 2; 1 patient discontinued before receiving study treatment, and 1 patient withdrew consent after cycle 1). Demographics and baseline disease characteristics for all enrolled patients are listed in Table 1. Median age was 36 years (age range, 18-69 years), 45% of patients had primary refractory disease, and 31% experienced relapse within 1 year of frontline therapy. The majority of patients received Adriamycin (doxorubicin), bleomycin, vinblastine, and dacarbazine (ABVD) (n = 56 [90%]) as first-line treatment of HL.
Clinical activity
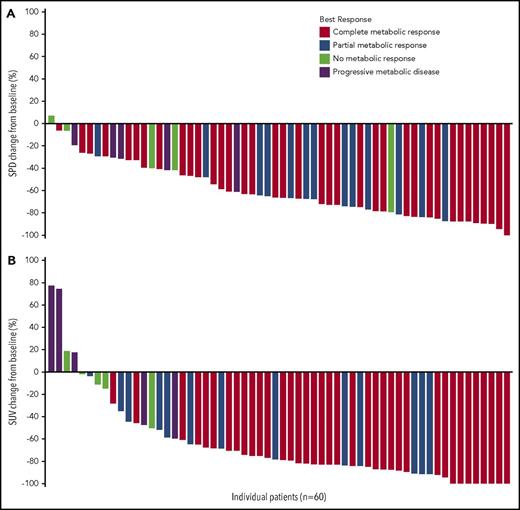
Response to study treatment was assessed at cycle 4 for all efficacy-evaluable patients, with the exception of 1 patient who was removed from treatment after cycle 2 with an assessment of stable disease (SD). Response rates and Deauville 5-point score for all treated patients (n = 61) and efficacy-evaluable patients (n = 60) are presented in Table 2. The CR rate among all treated patients was 61% (95% CI, 47%-73%), with an ORR of 82% (95% CI, 70%-91%). Among efficacy-evaluable patients, the CR rate was 62% (95% CI, 48%-74%), with an ORR of 83% (95% CI, 72%-92%). A decrease in tumor volume and metabolic activity was observed in 98% and 93% of efficacy-evaluable patients, respectively (Figure 1). One patient had a residual FDG-avid lesion (Deauville score of 5) after study treatment but was considered as having a CR because a biopsy showed no evidence of HL. Five patients (8%) experienced SD, and 5 patients (8%) progressed while receiving treatment. Subgroup analyses, which include response rates according to response to frontline therapy, are summarized in supplemental Table 1 and 2, available on the Blood Web site. No significant differences were observed in any of the subgroups analyzed.
Percent change in the sum of the product of diameters and maximum percent change in the standard uptake value in efficacy-evaluable patients (n = 60). (A) Sum of the product of diameters (SPD) percent change and (B) maximum standard uptake value (SUV) percent change are calculated as the percent change from the baseline SPD/SUV to the minimum post-baseline SPD/SUV measured before initiation of subsequent anticancer treatment (chemotherapy or radiotherapy, including conditioning regimen for ASCT).
Percent change in the sum of the product of diameters and maximum percent change in the standard uptake value in efficacy-evaluable patients (n = 60). (A) Sum of the product of diameters (SPD) percent change and (B) maximum standard uptake value (SUV) percent change are calculated as the percent change from the baseline SPD/SUV to the minimum post-baseline SPD/SUV measured before initiation of subsequent anticancer treatment (chemotherapy or radiotherapy, including conditioning regimen for ASCT).
Seventeen patients received salvage therapy subsequent to study treatment, among whom 12 patients received ICE, and 1 patient each received single-agent Nivo; single-agent BV; gemcitabine and oxaliplatin; bendamustine and BV; and bendamustine, gemcitabine, etoposide, and vinorelbine. The overall best response rate to poststudy alternative salvage chemotherapy was 80% (95% CI, 52%-96%), with a CR rate of 40% (95% CI, 16%-68%). Patient responses to each salvage therapy are summarized in supplemental Table 3. One patient required 4 lines of subsequent salvage therapy, including ICE, before proceeding to ASCT. Three patients had disease that was refractory to salvage therapy after treatment with BV and Nivo, all of whom had primary refractory disease.
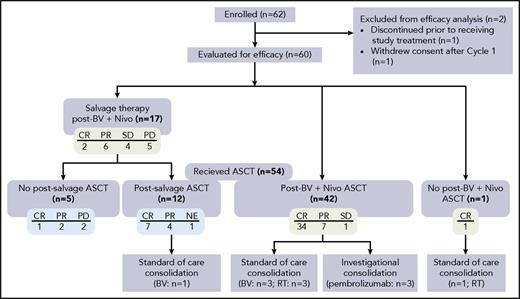
At the time of this analysis, 54 patients had undergone ASCT: 41 patients (76%) with CR, 11 patients (20%) with PR, and 1 patient (2%) with SD (1 patient was not evaluable) before transplantation (Figure 2). Forty-two patients underwent ASCT directly after treatment with BV and Nivo. Stem cell mobilization/engraftment data were available for 44 patients. Median time from EOT to the start of mobilization was 9 days (range, −12 to 50 days), including 1 patient who began mobilization 12 days before EOT. Mobilizing agents included granulocyte colony-stimulating factor (G-CSF) (n = 23), G-CSF with cyclophosphamide (n = 14), G-CSF with plerixafor (n = 5), or G-CSF with other combination chemotherapy (n = 2). A median of 4.7 × 106 CD34+ cells/kg was collected (range, 3-60 CD34+ cells/kg) in a median of 2 days of apheresis sessions. The conditioning regimens used before autologous stem cell infusion were carmustine, etoposide, cytarabine, and melphalan (n = 28); gemcitabine, vinorelbine, carmustine, etoposide, and cyclophosphamide (n = 8); carmustine, cyclophosphamide, and etoposide (n = 5); and other regimens (n = 3). Median times to neutrophil and platelet engraftment were 11.5 days (range, 8-29 days) and 16 days (range, 7-63 days), respectively.
Therapy after study treatment. Among the 60 patients evaluated for efficacy, 54 patients underwent ASCT, of whom 42 patients did so directly after treatment with BV and Nivo. A total of 17 patients received subsequent salvage therapy, and 1 patient who achieved CR received consolidation radiotherapy after treatment with BV and Nivo. Green boxes indicate response to BV plus Nivo, and blue boxes indicate response to salvage therapy. NE, not evaluable; PD, progressive disease; RT, radiation therapy.
Therapy after study treatment. Among the 60 patients evaluated for efficacy, 54 patients underwent ASCT, of whom 42 patients did so directly after treatment with BV and Nivo. A total of 17 patients received subsequent salvage therapy, and 1 patient who achieved CR received consolidation radiotherapy after treatment with BV and Nivo. Green boxes indicate response to BV plus Nivo, and blue boxes indicate response to salvage therapy. NE, not evaluable; PD, progressive disease; RT, radiation therapy.
One patient received consolidative radiotherapy after achieving CR to BV plus Nivo and did not proceed to transplantation. Among patients who proceeded to ASCT directly after BV plus Nivo, 3 patients (2 with CR and 1 with PR) underwent post-ASCT consolidative radiotherapy, 3 patients (all with PR) received consolidative BV, and 3 patients (2 with CR and 1 with PR) received pembrolizumab per investigational protocol. One patient with a CR to subsequent salvage therapy received consolidative BV after ASCT.
Median follow-up time was 7.8 months from the start of treatment (n = 61; range, 1-17 months) and 3.4 months from ASCT (n = 44; range; 1-12 months). Median DOR, which included the ASCT period as appropriate, was not reached. At 6 months, the median PFS rate for all patients had not been reached and the estimated PFS rate was 89% (95% CI, 75%-95%).
Safety
Sixty patients (98%) experienced treatment-emergent AEs before undergoing ASCT or receiving alternative salvage therapy. Among the most common events were nausea (49%), fatigue (41%), and infusion-related reactions (IRRs) (44%; Table 3). Grade 3 or higher events occurred in 19 patients (31%), with grade 3 anemia, febrile neutropenia, hypophosphatemia, and neutropenia occurring in 2 patients (3%) each. A total of 12 patients (20%) experienced treatment-emergent PN, 11 of whom had grade 1 symptoms. One patient who experienced grade 1 PN at baseline, and subsequently required a reduction in BV dose for a grade 2 event at cycle 2, discontinued study treatment after cycle 3 because of grade 3 PN. Treatment-related SAEs, which emerged before ASCT or salvage therapy, occurred in 6 patients (10%); these SAEs included pneumonitis, pneumonia, pyrexia, malaise, nausea, and rash.
IRRs, typically grade 1 or 2 in severity, occurred in 27 patients (44%), among whom 8 patients (30%) experienced their first IRR during the first cycle, 18 patients (67%) during cycle 2, and 1 patient (4%) during cycle 3. Twenty-five patients (41%) experienced an IRR during an infusion of BV. The most common IRR was grade 1 nausea (16%), and other associated symptoms included chest discomfort, urticaria, cough, flushing, and hypoxia. Two patients (3%) had grade 3 AEs during the BV infusion: 1 patient experienced pruritus and syncope, and another had urticaria. Because of the high rate of IRRs, we amended the study to institute mandatory premedication at cycles 2 to 4 (low-dose corticosteroids; hydrocortisone 100 mg or equivalent, and an antihistamine). Nonetheless, the rate of IRRs during cycle 2 was largely unchanged before and after premedication, whereas the rate of IRRs during cycles 3 to 4 remained low irrespective of premedication. No patients discontinued treatment because of an IRR. A summary of IRRs by grade is presented in supplemental Table 4.
Administrations of BV plus Nivo were delayed for 7 patients (11%) and 9 patients (15%), respectively, because of asymptomatic laboratory abnormalities that included elevated transaminase and lipase enzyme levels and neutropenia (n = 8); pneumonia and thrombosis (n = 2 each); and chills, pneumonitis, syncope, and urticaria (n = 1 each). BV administration was interrupted in 16 patients (26%), all because of IRRs.
With the exclusion of IRRs, AEs that were broadly categorized (according to AE term) as potentially immune related occurred in 50 patients (82%), among whom 14 patients (23%) had an IrAE possibly related to both study drugs. Five patients (8%) received systemic steroids for IrAEs, which included grade 3 diarrhea and grade 2 colitis (n = 1); grade 3 elevation of aspartate aminotransferase levels (n = 1); grade 4 colitis and grade 4 pneumonitis (n = 1, after receipt of additional salvage therapy); grade 2 pneumonitis (n = 1); and grade 4 pneumonitis (n = 1, after receipt of carmustine, etoposide, cytarabine, and melphalan as part of the ASCT-conditioning regimen). Among patients who did not receive steroids, grade 3 events included elevation of alanine aminotransferase levels (n = 1), diarrhea (n = 1), and maculopapular rash (n = 1). No patients discontinued treatment because of an IrAE.
Biomarker analyses
Peripheral blood immunophenotyping by flow cytometry
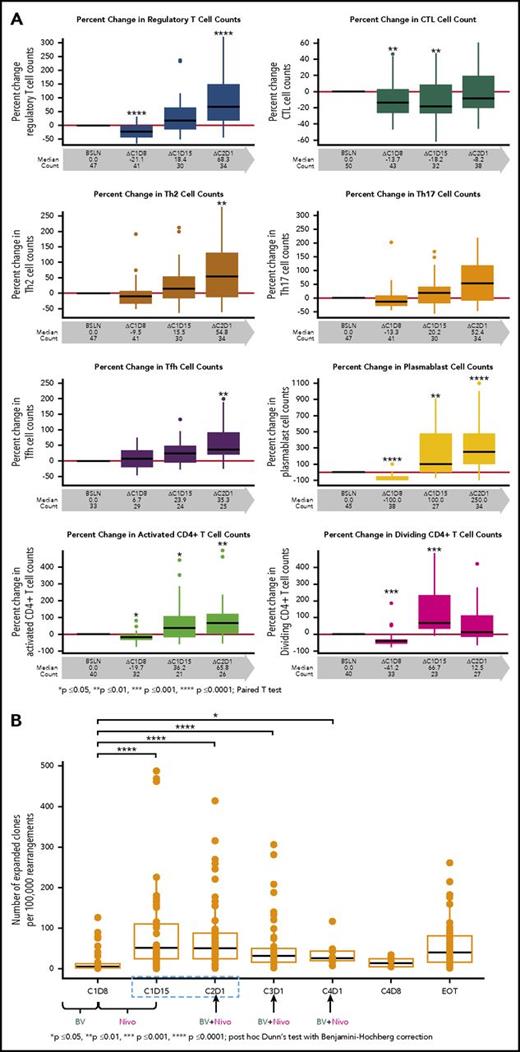
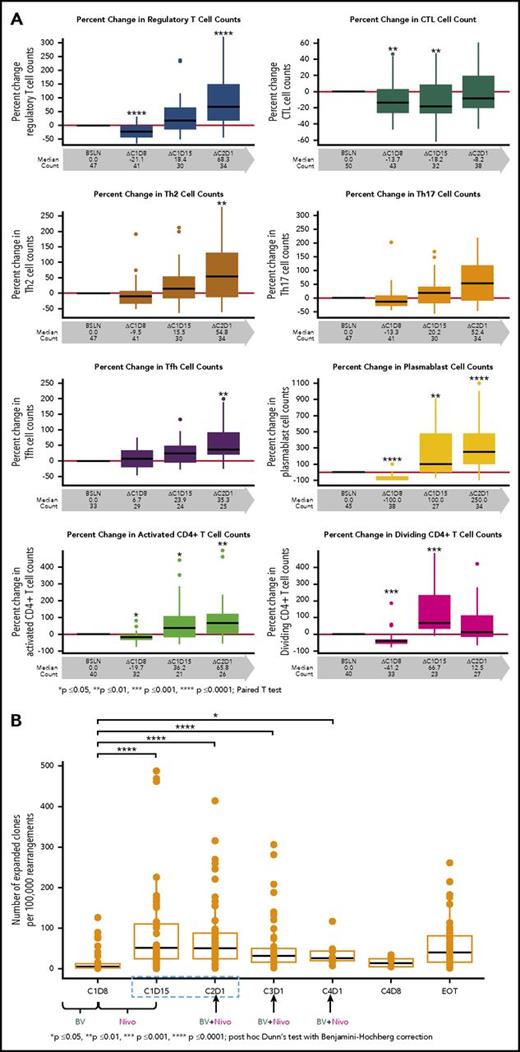
We evaluated the effects of the study drugs on circulating immune cells. The proportion of CD30-expressing cells among T- and B-cell subsets in the peripheral blood of all patients was assessed, and a higher percentage of regulatory T cells (Tregs) expressed CD30 compared with any other T-cell subset examined. Among B cells, a high percentage of plasmablasts expressed CD30 (data not shown). Tregs were significantly reduced in number after treatment with single-agent BV (Figure 3A, first panel). However, after Nivo treatment, Tregs significantly increased in number above baseline values. A similar trend was observed with plasmablasts as well as with T-cell subsets, which included activated and dividing CD4+ cells (including Tregs), T follicular helper cells, and Th2 and Th17 cells. CTLs were reduced in number in the periphery after single-agent BV treatment, and the numbers did not return to baseline until after C2D1 (Figure 3A, panels 2-8).
Flow cytometry results for immunophenotyping T-cell subsets and frequency of T-cell clones in the peripheral blood. (A) T-cell immunophenotyping included Tregs as defined by CD4+CD25+CD127low/−CCR4+; cytotoxic T cells (CTLs) as defined by CD8+; TH2 as defined by CD4+ CXCR3-CCR6-CCR4+; TH17 as defined by CD4+CXCR3-CCR6+CCR4+; T follicular helper (Tfh) CD4+CD45RA-CXCR3-CXCR5+; as well as for plasmablasts CD19+CD20-IgD-CD27+CD38hi. Activated and dividing CD4+ are defined by HLA-Dr and Ki67 expressions, respectively. (B) The frequency of T-cell clones per 100 000 clones is shown relative to baseline during the treatment course. P values were calculated for (A) and (B) using the paired t test (GraphPad Prism) and post hoc Dunn test with Benjamini-Hochberg correction, respectively.
Flow cytometry results for immunophenotyping T-cell subsets and frequency of T-cell clones in the peripheral blood. (A) T-cell immunophenotyping included Tregs as defined by CD4+CD25+CD127low/−CCR4+; cytotoxic T cells (CTLs) as defined by CD8+; TH2 as defined by CD4+ CXCR3-CCR6-CCR4+; TH17 as defined by CD4+CXCR3-CCR6+CCR4+; T follicular helper (Tfh) CD4+CD45RA-CXCR3-CXCR5+; as well as for plasmablasts CD19+CD20-IgD-CD27+CD38hi. Activated and dividing CD4+ are defined by HLA-Dr and Ki67 expressions, respectively. (B) The frequency of T-cell clones per 100 000 clones is shown relative to baseline during the treatment course. P values were calculated for (A) and (B) using the paired t test (GraphPad Prism) and post hoc Dunn test with Benjamini-Hochberg correction, respectively.
TCR clonality investigation by high-throughput TCR sequencing
We evaluated the impact of BV and BV plus Nivo on peripheral blood TCR clonality and T-cell clonal expansion. Although the TCR clonality in the periphery did not significantly change with study treatment, T-cell clonal expansion (increasing frequency of preexisting T-cell clones) was observed after treatment with BV and Nivo, which was concurrent with the T-cell subset elevation measured by flow cytometry (Figure 3B).
Serum cytokine and chemokine quantification
Among all patients, the first dose of BV resulted in elevation in proinflammatory cytokine and chemokine levels, and a concurrent reduction in serum thymus- and activation-regulated chemokine (TARC) levels, with these results maintained after treatment with Nivo. A significant increase in cytokine and chemokine levels associated with adaptive immune system activation was observed after treatment with BV and Nivo (Figure 4A). At baseline, IFN-γ–induced protein 10 (IP-10) levels were significantly lower (P = .0178, Wilcoxon rank-sum test with Benjamini-Hochberg multiple test correction) in patients who achieved CR vs patients who did not achieve CR. At baseline and subsequent time points, patients with CR had lower TARC levels than did patients without CR, although the differences were not significant (Figure 4B).
Longitudinal changes of cytokine levels during the treatment course and intracellular detection of IFN-γ in CD8+effector memory (CD45RA-CCR7−) cells. (A) Average cytokine levels (normalized ratio against baseline) of all patients are depicted by a heat map. Left box highlights proinflammatory monocyte chemokines including MCP-1, MCP-2, and proinflammatory cytokines including interferon-γ (IFN-γ) and INF-α. Middle boxes highlight proinflammatory T-cell chemokines including IFN-induced protein 10 (IP-10), ITAC, Mip-1b, and proinflammatory B-cell activators including BAFF and APRIL. Lower-right box shows cytokines released from RS cells (interleukin [IL]-10, TARC, and IL-6) that have been reported as negative prognostic factors. Levels of (B) IP-10 and TARC during the treatment course were analyzed by best response. Because of the small number of patients, data from C4D1 are excluded from the plots. (C) Intracellular cytokine staining of ex vivo peptide–stimulated T cells. Red box highlights the time point at which the largest separation between peptide antigen and control was observed. Staphylococcal enterotoxin B was used as a positive control, and nonstimulated (NS) was used as a negative control. EBV represents a peptide pool of Epstein-Barr virus–associated peptides, CEFT represents a peptide pool from Cytomegalovirus, Epstein-Barr virus, Influenza, and Tetanus toxin. Stimulation is indicated by red dots, and NS is depicted by blue dots. NR, no response including SD and progressive disease.
Longitudinal changes of cytokine levels during the treatment course and intracellular detection of IFN-γ in CD8+effector memory (CD45RA-CCR7−) cells. (A) Average cytokine levels (normalized ratio against baseline) of all patients are depicted by a heat map. Left box highlights proinflammatory monocyte chemokines including MCP-1, MCP-2, and proinflammatory cytokines including interferon-γ (IFN-γ) and INF-α. Middle boxes highlight proinflammatory T-cell chemokines including IFN-induced protein 10 (IP-10), ITAC, Mip-1b, and proinflammatory B-cell activators including BAFF and APRIL. Lower-right box shows cytokines released from RS cells (interleukin [IL]-10, TARC, and IL-6) that have been reported as negative prognostic factors. Levels of (B) IP-10 and TARC during the treatment course were analyzed by best response. Because of the small number of patients, data from C4D1 are excluded from the plots. (C) Intracellular cytokine staining of ex vivo peptide–stimulated T cells. Red box highlights the time point at which the largest separation between peptide antigen and control was observed. Staphylococcal enterotoxin B was used as a positive control, and nonstimulated (NS) was used as a negative control. EBV represents a peptide pool of Epstein-Barr virus–associated peptides, CEFT represents a peptide pool from Cytomegalovirus, Epstein-Barr virus, Influenza, and Tetanus toxin. Stimulation is indicated by red dots, and NS is depicted by blue dots. NR, no response including SD and progressive disease.
Intracellular cytokine staining of ex vivo peptide–stimulated T cells
Patients with R/R HL may have exhausted or immunosuppressed T cells that are not able to mount an appropriate response to antigens. In order to ascertain if BV or BV plus Nivo could reactivate exhausted or immunosuppressed T cells, we stimulated PBMCs ex vivo with pools of major histocompatibility complex (MHC) I and MHC II antigen peptides. This ex vivo peptide stimulation revealed the enhanced ability of T-cell subsets to respond to MHC I and MHC II antigens after treatment with BV and Nivo compared with baseline. Effector memory CD8+ T cells from some patients displayed increased intracellular interleukin-2 and IFN-γ after stimulation with MHC I and MHC II peptide pools compared with baseline. This potentially indicates elevated activation status of the immune system after combination treatment (Figure 4C), with the largest separation between peptide antigen and control occurring at C1D15, which is the same time point for which we observed peak clonal expansion and increase in circulating T-cell numbers.
Discussion
Among patients with R/R HL, the combination of BV plus Nivo as first salvage therapy was well tolerated and highly active. The ORR and CR rate on this study were 82% and 61%, respectively, with use of an outpatient regimen free of traditional combination chemotherapy. For the patients who did not achieve a CR, most of them were able to respond to subsequent therapies and proceed with ASCT.
The frequency and severity of AEs were similar to those observed with each agent administered individually, with the exception of the relatively higher proportion of patients who experienced IRRs. IRRs occurred most commonly during the second cycle of study therapy and were nearly always mild in severity; no patients discontinued study therapy because of an IRR. The etiology for the increased incidence of IRRs is unclear. Because IRRs have been observed with both agents (ADCETRIS [BV] prescribing information, Seattle Genetics, Inc, November 2017; OPDIVO [Nivo] prescribing information, Bristol-Myers Squibb, October 2017) and prior studies have evaluated patients at second or subsequent relapses who were more heavily pretreated, it is possible that patients in the first salvage setting have an increased ability to mount an immune response. The rate and severity of IRRs, however, did not substantially change with corticosteroid and antihistamine premedication. Although potential IrAEs were observed, only 8% of patients required treatment with systemic corticosteroids, but longer follow-up is needed to evaluate late immune-related events. In addition, there was no appreciable impact of study therapy on stem cell mobilization and collection yields or engraftment, and there was no signal of increased or unusual toxicities after ASCT in the patients who underwent ASCT, although again, longer follow-up is needed.
Peripheral blood biomarkers reflected immune activation after BV and BV plus Nivo therapy. Before treatment, Tregs had the highest proportion of CD30 positivity of any T-cell subset. Treatment with BV resulted in an initial reduction in T-cell subsets, including Tregs, and plasmablasts, as well as a reduction in serum TARC levels concurrent with a significant increase in proinflammatory cytokines and chemokines, including IP-10. We observed that patients who achieved a CR had significantly lower baseline serum IP-10 levels than did patients whose best response was not a CR. The cytokine/chemokine patterns, already established after single-agent BV, may reflect BV-induced depletion of CD30-positive, TARC-secreting RS cells and BV-mediated immune activation and induction of immunogenic cell death. After administration of Nivo, T-cell subset numbers in the periphery increased significantly, with the exception of CTLs, which did not approach baseline levels until after C2D1. Future evaluation of post-treatment tumor biopsies will be necessary to assess whether CTLs were homing to the microenvironment and a concurrent increase in tumor-infiltrating CTLs is observed.
Expansion of preexisting T-cell clones in the periphery was observed at concurrent time points as the peak increase in T-cell subsets. This clonal expansion of peripheral blood T cells has not been previously observed after anti–PD-1 administration alone. It is possible that BV modulates the tumor microenvironment (eg, release of increased or alternative tumor antigens, impact on immune cell subsets) and promotes T-cell clonal expansion after Nivo treatment. In addition, peak T-cell clone expansion and the increase in circulating T-cell numbers occurred at the same time points at which we observed the enhanced ability of T cells to mount a response to MHC I and MHC II antigens. It is important that further studies of tumor tissue before and after combination therapy will be necessary to determine whether observations in the tumor microenvironment mirror the changes seen in the peripheral blood.
Our results are promising in the context of other salvage therapy studies in R/R HL, a setting in which no randomized studies have been performed to define a single standard-of-care regimen before ASCT. The ORR and CR rate on this study are higher than those observed with BV or Nivo alone in R/R HL and are higher than with BV administered as initial salvage therapy. The rates that we observed are similar to those observed with sequential BV, followed by ICE or other salvage chemotherapy.31,32 Our response data are also similar to those reported in studies of multiagent salvage chemotherapy regimens,6,9-12,40,41 as well as studies combining BV with salvage chemotherapy.42-44 Because of the indeterminate responses and different kinetics of response observed with immunotherapy,45 a simple comparison of response rates or Deauville scores with chemotherapy-based salvage regimens may not be appropriate. Indeed, 1 patient in our study underwent a Deauville 5 PET scan at EOT, but biopsy results of the residual metabolically active area revealed no evidence of HL. Long-term follow-up of the durability of disease control will be critical in assessing the full therapeutic impact of combination BV plus Nivo therapy. It is important to note that the responses were achieved in an outpatient regimen, with nausea, fatigue, and IRRs as the most frequent AEs, which are distinct from the hematologic and nonhematologic toxicities expected with traditional chemotherapy-based salvage regimens. Increased exposure to chemotherapy is associated with organ and bone marrow toxicities, and long-term risks of secondary malignancies (including myelodysplasia and leukemia). Therefore, minimizing chemotherapy exposure in patients with R/R HL is appealing, both in young patients who often experience the downstream negative health consequences related to therapy for HL, and in older patients who may have comorbidities and in whom it would be desirable to minimize cumulative toxicity before ASCT.
The study cohort represents a typical population of transplant-eligible patients with HL who are receiving first salvage therapy. The proportion of patients who had early-stage disease at diagnosis was higher (60%) than the proportion with advanced-stage disease, but nearly half of patients had primary refractory disease (45%), which is a known risk factor for second-line treatment failure. We observed that all patients who had primary PD with study treatment had a history of primary refractory HL, and primary refractory patients (55%) appeared to have lower ORRs and CR rates (ORR, 69% vs 95%; CR, 50% vs 71%) compared with patients with relapsed disease, although no conclusions can be drawn because the sample size was small and the results were not significant. Lower response rates have been observed in primary refractory patients who were receiving salvage chemotherapy21 ; however, lower response rates have not been observed in primary refractory patients treated with sequential BV and salvage chemotherapy31,32 or in refractory patients treated with PD-1 inhibitors.46 Of note, patients who were enrolled in the present study were naive to prior BV and prior Nivo. Although re-treatment with BV can result in objective responses,47 it is unclear how effective the study regimen would be as first salvage therapy in patients who previously received BV or Nivo, or how it would influence the effectiveness of post-ASCT BV consolidation.
BV plus Nivo administered in combination as first salvage therapy for R/R HL was a tolerable and effective bridge to ASCT that replaced the need for cytotoxic chemotherapy for the majority of patients. A higher proportion of patients achieved metabolic CR compared with previous data on either BV or Nivo alone, a critical prognostic factor for ASCT outcome. Although durable responses after ASCT have been demonstrated in previous BV-based salvage regimens, additional follow-up will be necessary to assess the impact of the BV plus Nivo study regimen on long-term outcomes.31,32 The tolerability and activity of BV plus Nivo demonstrated in our study support further evaluation of this combination. An ongoing clinical trial is evaluating the combination of BV plus Nivo in patients with R/R HL who are ineligible for ASCT or who experience failure after ASCT (CheckMate 812, NCT03138499).
Presented in part at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 11 December 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to acknowledge Abraham Fong and Neil Josephson for their contribution to the design of the study, and analysis and interpretation of the data, and Katrina Sebolt for assistance in manuscript preparation, as employees of Seattle Genetics, Inc. The authors also thank the patients who participated in this study, and their families and caregivers.
This work was supported by the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award and the National Cancer Institute, National Institutes of Health (2K12CA001727 and P50CA107399) (A.F.H.). Direct funding for this research was issued by Seattle Genetics, Inc, through the joint financial support of Seattle Genetics, Inc, and Bristol-Myers Squibb.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: A.F.H., K.F., C.A.O., D.T., Q.Z., and M.C. contributed to the analysis and interpretation of the data and wrote the manuscript; A.F.H., A.J.M., N.L.B., J.M.V., R.R., T.A.F., A.S.L., S.M.A., C.H.M., K.K., and R.H.A. contributed to the acquisition of the data; A.J.M., N.L.B., J.M.V., R.R., T.A.F., A.S.L., S.M.A., C.H.M., K.K., and R.H.A. critically reviewed the manuscript; and all authors contributed to the concept and design of the study and approved the final version of the manuscript.
Conflict-of-interest disclosure: K.F., C.A.O., D.T., Q.Z., and M.C. are employees of and receive equity ownership in Seattle Genetics, Inc. A.F.H. received research funding from Seattle Genetics, Bristol-Myers Squibb, MedImmune, Merck, Immune Design, Genentech, and Pharmacyclics. A.J.M. received research funding from Seattle Genetics. N.L.B. received research funding from Seattle Genetics, Janssen, Affimed, ImaginAb, KITE, Forty Seven, Pharmacyclics, Celgene, Bristol-Myers Squibb, AstraZeneca, Genentech, Merck, Millennium, Pfizer, Immune Design, and Novartis. J.M.V. received research funding from Seattle Genetics, Bristol-Myers Squibb, Onyx, Merck, KITE, Janssen, Celgene, Allos Therapeutics, Acerta, Incyte, and US Biotest. R.R., T.A.F., and A.S.L. received research funding from Seattle Genetics. S.M.A. received research funding from Seattle Genetics and Merck. C.H.M. received research funding from Seattle Genetics, Pharmacyclics, and Merck. R.H.A. received research funding from Seattle Genetics, Agensys, Bristol-Myers Squibb, Celgene, Genentech, Infinity, Kura, Merck, Millennium, Regeneron, Janssen, and Pharmacyclics. A.F.H. has consulted for Merck, Genentech, Bristol-Myers Squibb, and Pharmacyclics. N.L.B. has consulted for Seattle Genetics, Gilead, and KITE. R.R. has consulted for Seattle Genetics. C.H.M. has consulted for Seattle Genetics, Celgene, Genentech, and Merck. R.H.A. has consulted for Gilead, Spectrum, Bristol-Myers Squibb, Pharmacyclics, NanoString, Forty Seven, Sutro, and Juno Therapeutics. T.A.F. is a member of a speakers bureau for Seattle Genetics, Pharmacyclics, Johnson & Johnson, AbbVie, and Celgene. The remaining author declares no competing financial interests.
The current affiliation for K.K. is the Division of Hematology/Oncology, Global Clinical R&D, Celgene Corporation, Summit, NJ.
Correspondence: Alex F. Herrera, Department of Hematology/Hematopoietic Cell Transplantation, City of Hope Medical Center, Duarte, CA; e-mail: aherrera@coh.org.


![Figure 4. Longitudinal changes of cytokine levels during the treatment course and intracellular detection of IFN-γ in CD8+ effector memory (CD45RA-CCR7−) cells. (A) Average cytokine levels (normalized ratio against baseline) of all patients are depicted by a heat map. Left box highlights proinflammatory monocyte chemokines including MCP-1, MCP-2, and proinflammatory cytokines including interferon-γ (IFN-γ) and INF-α. Middle boxes highlight proinflammatory T-cell chemokines including IFN-induced protein 10 (IP-10), ITAC, Mip-1b, and proinflammatory B-cell activators including BAFF and APRIL. Lower-right box shows cytokines released from RS cells (interleukin [IL]-10, TARC, and IL-6) that have been reported as negative prognostic factors. Levels of (B) IP-10 and TARC during the treatment course were analyzed by best response. Because of the small number of patients, data from C4D1 are excluded from the plots. (C) Intracellular cytokine staining of ex vivo peptide–stimulated T cells. Red box highlights the time point at which the largest separation between peptide antigen and control was observed. Staphylococcal enterotoxin B was used as a positive control, and nonstimulated (NS) was used as a negative control. EBV represents a peptide pool of Epstein-Barr virus–associated peptides, CEFT represents a peptide pool from Cytomegalovirus, Epstein-Barr virus, Influenza, and Tetanus toxin. Stimulation is indicated by red dots, and NS is depicted by blue dots. NR, no response including SD and progressive disease.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/11/10.1182_blood-2017-10-811224/4/m_blood811224f4.jpeg?Expires=1767883574&Signature=Pu7bwfDsV-N9RZS8zqcYjJnsiupbV0DMwZbb8WGch9AhenkWc-o7ggoT7cYUipfMGgBQ8vWMifWfqzxeIFK4~L2Zcfnfln31hjTznPEVlYNuqb6e~VOgNm-1GR2ESzqWPLq6QA5ko7aNCbnXo36IA8l69YaDEF03XkKjNXMtjpw84~ZP97n1x-dCAL8LH9iE~cZhwr~mTxokX7Y5ihtH6Hezw174djko-F5FY45awJL65BogO4PNpfhS82c0h89qxtXDwXUrwdlB~sPuCIwgP9BJhiCuKoOxgvQO~K6sXgKC5bUsKBsmNy15l1D8RHNNO7FTkXQO~BFbeA4VJYqjLw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 4. Longitudinal changes of cytokine levels during the treatment course and intracellular detection of IFN-γ in CD8+ effector memory (CD45RA-CCR7−) cells. (A) Average cytokine levels (normalized ratio against baseline) of all patients are depicted by a heat map. Left box highlights proinflammatory monocyte chemokines including MCP-1, MCP-2, and proinflammatory cytokines including interferon-γ (IFN-γ) and INF-α. Middle boxes highlight proinflammatory T-cell chemokines including IFN-induced protein 10 (IP-10), ITAC, Mip-1b, and proinflammatory B-cell activators including BAFF and APRIL. Lower-right box shows cytokines released from RS cells (interleukin [IL]-10, TARC, and IL-6) that have been reported as negative prognostic factors. Levels of (B) IP-10 and TARC during the treatment course were analyzed by best response. Because of the small number of patients, data from C4D1 are excluded from the plots. (C) Intracellular cytokine staining of ex vivo peptide–stimulated T cells. Red box highlights the time point at which the largest separation between peptide antigen and control was observed. Staphylococcal enterotoxin B was used as a positive control, and nonstimulated (NS) was used as a negative control. EBV represents a peptide pool of Epstein-Barr virus–associated peptides, CEFT represents a peptide pool from Cytomegalovirus, Epstein-Barr virus, Influenza, and Tetanus toxin. Stimulation is indicated by red dots, and NS is depicted by blue dots. NR, no response including SD and progressive disease.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/11/10.1182_blood-2017-10-811224/4/m_blood811224f4.jpeg?Expires=1767883575&Signature=wsb6wi3QuXiO30VlZX05uH82ldQqjdzTcLTDHcMimcudr00kiWYhR5xtu66tnXJ8FRpSeiLCZoQu0wQ7TxKcwzGY6UeMyKV690Oc0Uogua60REHCELMALV4ATAGoW4VzmIC1JBmV2eJuSUNXjpnk5AR1vOHawxNMH7~8tg2veteW7nnMqKl0tW0N5eoV~ZkSwQ7xNh4NZ-7~vuH9VBMtVXI9b5o6rSkE3mvmVTNn4obI0u8QFNL9wKw7zmZIFd2BhouYkoBcmnW3OKsryO-GGvd2rK-NGb7YMrJ46GfDlbf7NWe9jbId9XRWteAK54tv9IRlXOV7vwtD80J-2~PXxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)