Abstract
Background: Frailty is a state of depleted physiologic reserve that is common in older patients with myeloid malignancies. While it is associated with chemotherapy-related toxicity, poor response to therapy, and higher mortality (Sherman, 2013; Fega, 2015), little is known about its causes. Moreover, there are increasing data demonstrating that clonal somatic mutations may be related to atherosclerotic disease (Jaiswal, 2017), which is an important driver of frailty. In this context, we sought to determine whether there is an association between hematopoietic DNA mutations and frailty in patients with overt myeloid neoplasms, hypothesizing that both RAS/MAPK and spliceosome mutations would be associated with the frailty phenotype.
Methods: Starting in February of 2015,all patients aged 75 and older who presented for initial consultation to the DFCI Adult Leukemia Clinic were approached for evaluation by a trained research assistant. In a 15-minute interview, the assistant used two methods to characterize the patient as frail, pre-frail, or robust. The first employs a cumulative deficit approach (Rockwood, 2007) including 26 questions adapted from the Yale Precipitating Events Project (Searle, 2008), as well as the delayed recall section of the Montreal Cognitive Assessment (Nasreddine, 2005), a clock-in-the-box test (Chester, 2011), a grip strength test (Gill, 2006), and a gait speed test (Studenski, 2011). The second is a phenotype approach (Fried, 2001), which gives equal weight to performance on the gait speed and grip strength tests, as well as three questions about weight loss, energy expenditure and self-reported exhaustion. Patients were assigned frailty designations using the worse of the two scores. 85% of patients approached agreed to participate. Routine assessment of recurrent genetic alterations was performed using the Rapid Heme Panel (RHP), a gene panel sequencing test that evaluates 95 genes recurrently mutated in hematologic malignancies (Kluk, 2016). Associations between mutations and frailty were assessed via tests for trend across frailty status categories as well as a Fisher's exact test comparing the proportion of patients with robust status (versus frail/pre-frail combined).
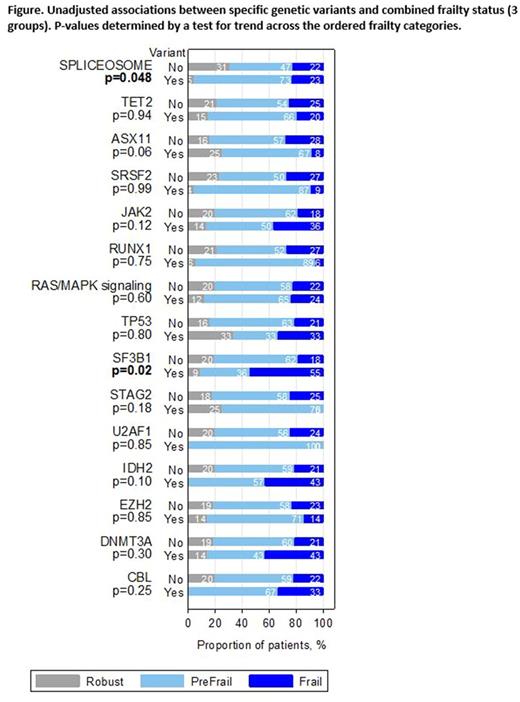
Results: As of July 2017, 93 patients with MDS (n=39), MPN (n=18), MDS/MPN (n=18), or AML (n=18) had completed frailty assessment and disease genetic characterization. Patients were majority male (n=70, 75%) with median age of 78 years (range, 75-89). Hypertension (65%) and hyperlipidemia (42%) were common. 59% (n=55) of patients in the cohort were pre-frail, 23% were frail (n=21), and 18% (n=17) were robust. There were no significant differences in frailty status between AML patients and those with chronic myeloid disorders (MDS, MPN, MDS/MPN). Gene mutations present in at least 5% of the cohort included SRSF2 (25%), SF3B1 (12%) , U2AF1 (8%), TET2 (44%), ASXL1 (26%), DNMT3A (8%), JAK2 (24%), RUNX1 (19%), TP53 (13%), STAG2 (9%), IDH2 (8%), EZH2 (8%) , and CBL (6%). 47% of patients had one of 4 non-co-occurring splicing mutations (SRSF2, SF3B1, U2AF1, ZRSR2). 18% of patients had one or more mutations that cause aberrant activation of RAS/MAPK signaling (NRAS, KRAS, CBL, PTPN11, RIT1, FLT3, KIT) and are associated with leukemic transformation of MDS (Lindsley, 2015). Patient with any splicing mutations were less likely to be robust (5% robust, 73% pre-frail, 23% frail) compared to those without (31% robust, 47% pre-frail, 22% frail; P for trend = 0.05; P -exact for robust versus pre-frail/frail = 0.001). Patients with an SF3B1 mutation specifically were also less likely to be robust then patients without an SF3B1 mutation (P for trend=0.02, Figure). The association between presence of a splicing mutation and frailty persisted within subgroups of patients with chronic myeloid malignancies and patients with AML, although the latter did not reach statistical significance (p=0.10) in that small dataset. No other single mutation, number of mutations present, or presence of a mutation in the RAS/MAPK pathway was significantly associated with frailty.
Conclusion: In our cohort, patients with splicing mutations were less likely to be robust than patients without splicing mutations. These findings suggest that molecular characterization of myeloid disease may identify patients at higher risk for frailty and complications associated with treatment.
DeAngelo: Glycomimetics: Research Funding; Amgen: Consultancy, Research Funding; BMS: Consultancy; Blueprint Medicines: Honoraria, Research Funding; Immunogen: Honoraria, Research Funding; Shire: Honoraria; Takeda Pharmaceuticals U.S.A., Inc.: Honoraria; Celgene: Research Funding; Incyte: Consultancy, Honoraria; Pfizer Inc.: Consultancy, Honoraria, Research Funding; ARIAD: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding. Steensma: Celgene: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Janssen: Consultancy, Research Funding; H3 Biosciences: Consultancy; Incyte: Equity Ownership; Onconova: Consultancy; Takeda: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lane: Stemline Therapeutics: Research Funding; N-of-one: Consultancy. Lindsley: MedImmune: Research Funding; Jazz Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.