To the editor:
We have shown that it is feasible to administer a first-generation tyrosine kinase inhibitor (TKI) early after allogeneic hematopoietic cell transplantation (HCT) in patients with Philadelphia chromosome-positive (Ph+) leukemia.1 The European Society for Blood and Marrow Transplantation recommends imatinib as the first choice for posttransplant relapse prevention because the data overall support lower relapse rates and improved survival.2 Current treatment paradigms favor early HCT for chronic myelogenous leukemia (CML) presenting in blast crisis or accelerated phase, or for adults with Ph+ acute lymphoblastic leukemia (ALL) in first remission. However, for chronic phase CML or in children with Ph+ ALL, effective TKI-based therapy has led to HCT being reserved for TKI resistance and/or intolerance.3,4 This limiting of HCT to the highest-risk Ph+ leukemias makes the problem of posttransplant relapse even more relevant than before the imatinib era. In the current follow-up study, we asked whether posttransplant nilotinib could be used to improve outcomes for patients with imatinib intolerance (I-I) or imatinib-resistant leukemia (I-RL). Previous non-HCT trials showed that nilotinib and imatinib had comparable hematopoietic toxicity, but edema and gastrointestinal toxicity occurred less commonly with nilotinib.5
Between September 2008 and September 2013, 57 patients, mostly adult, consented before HCT to participate in a single-arm feasibility study of posttransplant nilotinib therapy. Given nilotinib’s unique QTc prolongation risks and data showing that imatinib was tolerable early after engraftment, relapse prophylaxis began with imatinib until posttransplant day 80 except in patients with I-I or I-RL, where risk-benefit considerations dictated starting with nilotinib. The primary feasibility endpoint defined individual success as receipt of nilotinib ≥400 mg/d for ≥85% of the interval between day 81 and day 365, although 1 full year of nilotinib was planned through day 445. All grade 3 or 4 adverse events (AEs) were collected and graded according to Common Terminology Criteria for Adverse Events version 3.0, and overall study success was prespecified as 75% of subjects being individually successful. The primary efficacy endpoint defined individual success as complete molecular remission at 1 year posttransplant. The study was approved by the institutional review boards of the participating institutions.
Major inclusion criteria were as follows: allogeneic HCT recipients of any age with BCR/ABL-expressing ALL or CML, body surface area ≥1 m2, and treated with myeloablative conditioning. Patients with CML were in first chronic phase if age ≤17 years or second chronic phase. Any patient with CML chronic phase and pretransplant rising minimal residual disease by molecular (or other methods) despite TKI therapy was eligible, in which case BCR/ABL mutational analysis was obtained, but the results did not affect eligibility. Major exclusion criteria for starting nilotinib with engraftment or at day 81 were as follows: myocardial infarction ≤1 year prior, congestive heart failure, uncontrolled hypertension, unstable angina, complete left bundle branch block or bifascicular block, use of a ventricular-paced pacemaker, congenital or family history of long QT syndrome, significant ventricular or atrial tachyarrhythmias, resting heart rate <50 beats per minute, and Fridericia corrected QT (QTcF) interval >450 milliseconds despite correction of abnormal serum electrolyte levels before the first dose of nilotinib.
After engraftment, subjects with imatinib-sensitive leukemia (I-SL) began treatment with imatinib at 400 mg (adults) or 260 mg/m2 (children) once daily when all eligibility criteria were satisfied. From day 81, subjects with I-SL switched to nilotinib therapy, 400 mg (children 230 mg/m2) once to twice daily depending on tolerability, for 365 days. In contrast, subjects with I-I or I-RL started nilotinib before day 80 at 300 mg (175 mg/m2) once to twice daily, with a plan to increase to higher dosing at day 81. Before day 80, imatinib and nilotinib dose reductions were planned for absolute neutrophil count <1.0 × 109/L not responsive to filgrastim, platelet count <10 × 109/L, serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) ≥5 times upper limit of normal, or conjugated bilirubin >3 times upper limit of normal. After day 80, filgrastim administration was optional, and the platelet threshold for holding nilotinib therapy was <15 × 109/L. Nilotinib was held for grade 3 or higher lipase or amylase elevations, or grade 2 or higher pancreatitis until asymptomatic with grade 0 to 1 lipase and amylase elevations according to modified (for HCT) National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Electrocardiographic evaluations of QTc were done at baseline and 8 days after starting or changing nilotinib dose, or whenever a potentially QT-prolonging medication was added. Nilotinib was discontinued when QTcF was >480 milliseconds and not correctable with potassium or magnesium supplements, removal of offending concomitant medication, or nilotinib dose reduction, in that order. A single recurrence of QTcF >480 milliseconds despite these measures led to therapy discontinuation and the designation “treatment failure.”
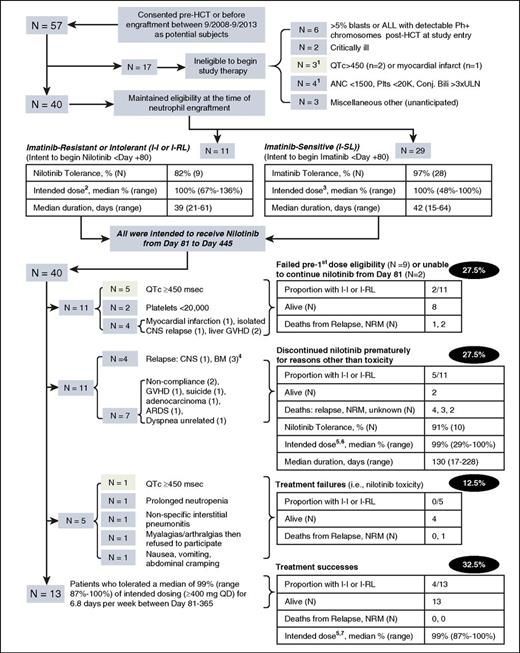
The flow and major study outcomes for all registered subjects are detailed in Figure 1. Critical to note are 2 “intent-to-treat” (ITT) populations: the first (N = 57), at time of consent, evolved into the second (N = 40), because 17 subjects lost eligibility to begin relapse prophylaxis at engraftment. Among this fully eligible ITT population, 95% had pretransplant molecular minimal residual disease. A second wave of discontinuations occurred for patients with I-SL at the day-81 switch to nilotinib, and additional discontinuations occurred after day 81. Overall, treatment failed in 77% (44 of 57) of the starting ITT population; 28 patients (49%) could not begin or continue nilotinib at day 81 because of posttransplant eligibility exclusions (frequently QTc >450 milliseconds [n = 7] or myocardial infarction [n = 2], early relapse [n = 8], or because of laboratory abnormality exclusions [n = 6]). A further 16 (28%) subjects discontinued nilotinib after day 81 because of definitive nilotinib toxicity (n = 5) or for reasons other than toxicity (n = 11).
Study flow and outcomes through 3 years of follow-up on all patients.1One of 17 patients ineligible to begin study therapy at engraftment had QTc >450 and absolute neutrophil count <1500. 2Median 300 mg twice daily (range 300 mg once daily to 300 mg twice daily), 6 to 7 days per week. 3Median 400 mg once daily, 6 to 7 days per week. 4Two subjects with I-SL per protocol switched to full-dose nilotinib at day 81; 1 later developed T315I and F311L mutations in the marrow at day 236, progressed, and died at day 638. The other developed the Y253H mutation at day 198, was switched to dasatinib, and survived. 5Intended dose ≥400 mg once daily (range 400 mg daily to 400 mg twice daily), from day 81 until day 445, 6 to 7 days per week. 6Five of these 11 patients tolerated 400 mg twice daily. 7Six of these 13 patients tolerated 400 mg twice daily. Pale green shaded boxes indicate 9 discontinuations due to QTc prolongation from the 57 subjects in the initial ITT population. ARDS, acute respiratory distress syndrome; BM, bone marrow; CNS, central nervous system; Conj. Bili, conjugated bilirubin; GVHD, graft-versus-host disease; NRM, nonrelapse mortality; Plts, platelets.
Study flow and outcomes through 3 years of follow-up on all patients.1One of 17 patients ineligible to begin study therapy at engraftment had QTc >450 and absolute neutrophil count <1500. 2Median 300 mg twice daily (range 300 mg once daily to 300 mg twice daily), 6 to 7 days per week. 3Median 400 mg once daily, 6 to 7 days per week. 4Two subjects with I-SL per protocol switched to full-dose nilotinib at day 81; 1 later developed T315I and F311L mutations in the marrow at day 236, progressed, and died at day 638. The other developed the Y253H mutation at day 198, was switched to dasatinib, and survived. 5Intended dose ≥400 mg once daily (range 400 mg daily to 400 mg twice daily), from day 81 until day 445, 6 to 7 days per week. 6Five of these 11 patients tolerated 400 mg twice daily. 7Six of these 13 patients tolerated 400 mg twice daily. Pale green shaded boxes indicate 9 discontinuations due to QTc prolongation from the 57 subjects in the initial ITT population. ARDS, acute respiratory distress syndrome; BM, bone marrow; CNS, central nervous system; Conj. Bili, conjugated bilirubin; GVHD, graft-versus-host disease; NRM, nonrelapse mortality; Plts, platelets.
Nine patients died, for a 1-year all-cause mortality of 22.5%. Twenty-one patients experienced serious AEs. One event of acute acalculous cholecystitis was unexpected. In another patient, nilotinib could have contributed to pericardial effusion in the context of septicemia. Thirty-one patients (78%) experienced a total of 110 AEs. The most frequent AEs were thrombocytopenia (47.5% of patients), lymphocytopenia (27.5%), elevated AST/ALT (25%), anemia (17.5%), neutropenia (17.5%), and cytomegalovirus viremia (17.5%). Anorexia, nausea, and diarrhea occurred in ≤5% of patients. Among the 43 (39%) AEs that were possibly (38), probably (3), or definitely (2) related to nilotinib therapy, 58% were grade 3, and 42% were grade 3 to 4. The most frequent related AEs were thrombocytopenia (16), neutropenia (8), anemia (7), and elevated AST/ALT (4).
This study demonstrates that among patients with I-I or I-RL, 82% tolerated nilotinib at an effective dose before day 81 (Table 1). We also confirmed our prior observation that imatinib can be delivered early after transplant at the intended dose intensity to almost all patients with I-SL. With a median of 4.15 years follow-up, 67.5% of the second ITT population were alive at 3 years, and 15% had relapsed with ≥3 years follow-up for all survivors (supplemental Table 1, available on the Blood Web site). It is of interest that all 13 subjects who completed nilotinib therapy at the target dose are alive without relapse. A larger prospective controlled trial would be necessary to confirm this apparent high rate of efficacy. Despite encouraging efficacy data in a study cohort whose relapse risk was high, our nilotinib relapse prophylaxis strategy was limited by QTc prolongation in 1 of 6 patients in the initial ITT population, or by other events that precluded beginning or continuing posttransplant nilotinib therapy. Shimoni et al were the first to report outcomes from a prospective nilotinib prophylaxis study. Their intended dose of 400 mg every 12 hours was not achievable, and the maximal tolerated dose was 200 mg every 12 hours. Similar to us, they observed 6 of 22 enrollees unable to even begin nilotinib because of the following: early death or disease progression, severe graft-versus-host disease, and patient refusal.6 Six of the remaining 16 (37.5%) stopped nilotinib prematurely because of the following: liver function abnormalities, hematological toxicity, late cerebrovascular event, or allergic reaction. Almost half of our study subjects (11 of 24) who were able to tolerate ≥400 mg once daily, including those who tolerated nilotinib for 1 full year or a median of 130 days for those who stopped early but not due to toxicity, in fact tolerated 400 mg twice daily. However, in practical terms, 23% of pretransplant patients could continue treatment with nilotinib for 12 months after HCT. We conclude that, although the role remains clear, optimizing TKI therapy for high-risk Ph+ leukemia after HCT remains challenging.
The online version of this article contains a data supplement.
This trial was registered at www.clinicaltrials.gov as #NCT00702403.
Authorship
Acknowledgment: This study was funded partly by an award from Novartis Pharmaceuticals (CAMN107AUS07T).
Contribution: P.A.C. was principal investigator, designed and wrote the study, and wrote the manuscript; M.J.M., H.F.F., and L.J. were collaborating site investigators with associated responsibilities; M.E.D.F. participated in study design discussions and treated patients at Fred Hutchinson Cancer Research Center; T.A.G. focused on statistical end points in the study design; and P.J.M., and J.P.R. participated in the study design discussion and critical analysis of the results and manuscript.
Conflict-of-interest disclosure: J.P.R. is on advisory boards for Novartis, Bristol Myers Squibb, Pfizer, and ARIAD and has a laboratory contract with Novartis. M.J.M. receives institutional research funding from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Paul A. Carpenter, Fred Hutchinson Cancer Research Center, Mailstop D5-290, 1100 Fairview Ave N, Seattle WA 98109-1024; e-mail: pcarpent@fredhutch.org.