Key Points
BAY 1143572, a novel and selective P-TEFb/CDK9 inhibitor, possessed significant antitumor activity against primary ATL cells in vitro.
BAY 1143572 possessed significant antitumor activity in an ATL mouse model based on tumor cells from a patient.
Abstract
Cyclin-dependent kinase 9 (CDK9), a subunit of the positive transcription elongation factor b (P-TEFb) complex, regulates gene transcription elongation by phosphorylating the C-terminal domain (CTD) of RNA polymerase II (RNAPII). The deregulation of CDK9/P-TEFb has important implications for many cancer types. BAY 1143572 is a novel and highly selective CDK9/P-TEFb inhibitor currently being investigated in phase 1 studies. We evaluated the therapeutic potential of BAY 1143572 in adult T-cell leukemia/lymphoma (ATL). As a result of CDK9 inhibition and subsequent inhibition of phosphorylation at serine 2 of the RNAPII CTD, BAY 1143572 decreased c-Myc and Mcl-1 levels in ATL-derived or human T-cell lymphotropic virus type-1 (HTLV-1)–transformed lines and primary ATL cells tested, leading to their growth inhibition and apoptosis. Median inhibitory concentrations for BAY 1143572 in ATL-derived or HTLV-1–transformed lines (n = 8), primary ATL cells (n = 11), and CD4+ cells from healthy volunteers (n = 5) were 0.535, 0.30, and 0.36 μM, respectively. Next, NOG mice were used as recipients of tumor cells from an ATL patient. BAY 1143572–treated ATL-bearing mice (once daily 12.5 mg/kg oral application) demonstrated significantly decreased ATL cell infiltration of the liver and bone marrow, as well as decreased human soluble interleukin-2 receptor levels in serum (reflecting the ATL tumor burden), compared with untreated mice (n = 8 for both). BAY 1143572–treated ATL-bearing mice demonstrated significantly prolonged survival compared with untreated ATL-bearing mice (n = 7 for both). Collectively, this study indicates that BAY 1143572 showed strong potential as a novel treatment of ATL.
Introduction
Adult T-cell leukemia/lymphoma (ATL), a peripheral T-cell neoplasm, is caused by human T-cell lymphotropic virus type-1 (HTLV-1).1-5 ATL is classified into 4 clinical subtypes, namely acute, lymphoma, chronic, and smoldering, according to criteria proposed by the Japan Lymphoma Study Group.6 The median survival time of ATL patients with acute and lymphoma subtypes (n = 807), diagnosed between 2000 and 2009 and who did not receive an allogeneic hematopoietic stem cell transplantation, was found to be 7.7 months,7 indicating that ATL currently has a poor prognosis.1 Although novel therapeutic agents have recently been reported for ATL patients,8-12 the development of additional treatment strategies for patients with ATL is an ongoing and urgent issue.
Cyclin-dependent kinase 9 (CDK9) is a serine (Ser)/threonine kinase, and forms a subunit of the positive transcription elongation factor b (P-TEFb) complex that regulates gene transcription elongation by phosphorylating the C-terminal domain (CTD) of RNA polymerase II (RNAPII).13 A deregulated CDK9-signaling system has important implications in the development and/or maintenance of a malignant cell phenotype. In this context, deregulation of CDK9-related pathways is found in many human malignancies.14-19 Additionally, CDK9-related pathways regulate the replication program of numerous viral agents, including HTLV-1.20,21
These findings prompted us to hypothesize that CDK9 may be a novel molecular target for the treatment of ATL. We have thus evaluated the therapeutic potential of a novel and highly selective inhibitor of CDK9/P-TEFb, BAY 1143572, for ATL.
Materials and methods
CDK9/P-TEFb inhibitor
BAY 1143572 (Bayer AG Pharmaceuticals Division, Berlin, Germany) is a selective inhibitor of CDK9/P-TEFb, currently being investigated in phase 1 studies in patients with advanced cancer (clinicaltrials.gov identifier: NCT01938638) or acute leukemia (clinicaltrials.gov identifier: NCT02345382). The 50% inhibitory concentrations (IC50) of BAY 1143572 for CDK9/cyclin T1 were 130-, 90-, 110-, 150-, 880-, and 20 000-fold lower than those for CDK1/cyclin B, CDK2/cyclin E, CDK3/cyclin E, CDK5/p35, CDK6/cyclin D3, and CDK7/cyclin H/MAT1, respectively.22
Cell lines
ATN-1, MJ, MT-1, and TL-Om1 are ATL-derived cell lines, whereas MT-2, MT-4, and TL-Su are HTLV-1–transformed cell lines; these 7 lines are all interleukin-2 (IL-2) independent, as previously described.23-25 S-YU is an ATL-derived line that can only be maintained by serial transplantation in immunodeficient mice but not by in vitro culture.26,27 HTLV-1–uninfected human T-cell acute lymphoid leukemia (T-ALL) cell lines, Jurkat, CCRF-CEM, and TALL-1, have been previously described.28
Primary ATL cells and control subjects
Primary ATL cells were isolated using anti-human CD4 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) from peripheral blood mononuclear cells (PBMCs) of 10 acute and 3 chronic subtype patients. The remaining primary ATL cells were isolated in the same manner from the affected lymph node of an acute subtype patient. Five healthy volunteers participated as controls, with their samples remaining anonymous and untraceable; CD4+ cells were isolated from their PBMCs in the same manner. All donors provided informed, written consent prior to sampling according to the Declaration of Helsinki. The present study was approved by the institutional ethics committees of the Graduate School of Medical Sciences, Nagoya City University.
Cell proliferation assay
The proliferation of ATL-derived or HTLV-1–transformed lines, other than S-YU and T-ALL, which could be maintained in in vitro culture in the presence of different concentrations of BAY 1143572 for 72 hours, was assessed using CellTiter 96 Aqueous One Solution cell proliferation assay kits (Promega Corporation, Madison, WI) as described previously.29 The proliferation of S-YU in the presence of 100 IU/mL recombinant human IL-2 (Miltenyi Biotec), with different concentrations of BAY 1143572 for 24 hours, was similarly assessed. The proliferation of primary ATL and CD4+ cells from healthy volunteers, which could not be maintained in in vitro culture for a long time, in the presence of 100 IU/mL recombinant IL-2 and with different concentrations of BAY 1143572 for 24 hours was also similarly assessed. All expressed values were averages of triplicate experiments.
In the present study, the IC50 was defined as the concentration of inhibitor where the response was reduced by half the maximal value. As an ad hoc analysis, the absolute IC50 was defined as the concentration of an inhibitor that reduced cell survival to 50% of the untreated control value, with the highest viability (no inhibitor) defined as 100%, and the lowest viability defined as 0%. Both values were calculated using GraphPad Prism 5 (GraphPad Software, San Diego, CA) according to the manufacturer’s instructions.
Quantitative reverse transcription polymerase chain reaction
ATL-derived or HTLV-1–transformed lines were incubated with various concentrations of BAY 1143572 for 5 hours. Quantitative reverse transcription polymerase chain reaction for HTLV-1 bZIP factor (HBZ) was performed as previously described.30
Apoptosis assay
The apoptosis of ATL-derived or HTLV-1–transformed lines, and T-ALL lines exposed to BAY 1143572 for 72 hours, was evaluated using the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. Cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences) with the aid of FlowJo software (Tree Star, Ashland, OR).
Western blotting
ATL-derived or HTLV-1–transformed lines and T-ALL lines were incubated with or without BAY 1143572 for 5 hours. Primary tumor cells from patients with ATL, and CD4+ cells from healthy volunteers, were incubated with or without BAY 1143572 for 12 hours, in the presence of 100 IU/mL recombinant IL-2. Whole-cell extracts were prepared and analyzed as previously described.31 Each loaded sample was adjusted to 30 μg per 10 μL, after estimating the total protein content using Bradford reagent (Sigma-Aldrich, St. Louis, MO). Antibodies to RNAPII (N-20), c-Myc, Mcl-1, and Actin were obtained from Santa Cruz Biotechnology (Dallas, TX). Antibodies to phosphorylated RNAPII (phospho-RNAPII) (Ser2 of the CTD [Ser2]) and RNAPII (Ser5) were obtained from Bethyl Laboratories (Montgomery, TX). HTLV-1 Tax expression was assessed by an anti-Tax monoclonal antibody (mAb), Lt-4.32 Proteins were visualized using goat anti-rabbit immunoglobulin G–horseradish peroxidase or goat anti-mouse immunoglobulin G–horseradish peroxidase (Santa Cruz Biotechnology).
Animals
NOD/Shi-scid, IL-2Rγnull (NOG) mice were purchased from the Central Institute for Experimental Animals (Kanagawa, Japan) and used between 6 and 8 weeks of age. All in vivo experiments were performed in accordance with the United Kingdom Coordinating Committee on Cancer Research Guidelines for the Welfare of Animals in Experimental Neoplasia (second edition) and were approved by the ethics committee of the Center for Experimental Animal Science, Graduate School of Medical Sciences, Nagoya City University.
ATL cell–bearing mice treated with BAY 1143572
A leukemic cell clone isolated from an ATL patient, S-YU, as reported previously,25,26,33 was injected intraperitoneal(ly) (i.p.) into NOG mice. Three to 4 weeks after i.p. injection, NOG mice developed i.p. masses within the mesentery. Cells from these i.p. masses were suspended in RPMI 1640 and inoculated i.p. into healthy NOG mice, which then presented with disease features identical to those of the original mice. ATL tumor cells (S-YU) from i.p. masses were suspended in RPMI 1640, and 1.0 × 107 cells were inoculated i.p. into each of 16 naive NOG mice. The animals were randomly divided into 2 groups of 8 each for treatment with BAY 1143572 or vehicle, 7 days after ATL cell inoculations. BAY 1143572 was formulated in 40% polyethylene glycol 400 in water. Mice were treated by oral application of 12.5 mg/kg BAY 1143572 (0.25 mg/100 μL per mouse) or vehicle (100 μL), once daily for 18 days (7-24 days after ATL cell inoculations). Antitumor activity was evaluated 25 days after ATL cell inoculations.
ATL cells from i.p. masses suspended in RPMI 1640 were inoculated i.p. into another 14 naive NOG mice at 1.0 × 107 cells per mouse. These animals were randomly divided into 2 groups of 7 each for treatment with BAY 1143572 or vehicle. BAY 1143572 was formulated in the same manner, and mice were treated by oral application of 12.5 mg/kg BAY 1143572 or vehicle, once daily for 21 days (7-27 days after ATL cell inoculations). The antitumor activity of BAY 1143572 was evaluated according to survival times.
ATL cells from i.p. masses suspended in RPMI 1640 were inoculated i.p. into another 6 naive NOG mice at 1.0 × 107 cells per mouse. These animals were randomly divided into 2 groups of 3. BAY 1143572 was formulated in the same manner, and mice were treated by oral application of 12.5 mg/kg BAY 1143572 or vehicle, once daily for 21 days (7-27 days after ATL cell inoculations). Twenty-eight days after ATL cell inoculations, i.p. masses were harvested, and western blotting analysis of protein extracts from tumor masses was performed.
Flow cytometry analysis of cells inoculated in mice
The following mAbs were used for flow cytometry: BD Multitest CD3/CD8/CD45/CD4 (BD Biosciences), allophycocyanin-conjugated anti-human CD45 (2D1; BD Biosciences), peridinin-chlorophyll-protein complex–conjugated anti-CD4 (SK3; BD Biosciences), phycoerythrin-conjugated anti-CD25 (M-A251; BD Biosciences), and the appropriate isotype control mAbs. Stained cells were analyzed on a FACSCalibur flow cytometer using FlowJo software.
Histological analysis
Soluble IL-2 receptor measurement
The concentration of human soluble IL-2 receptor (sIL2R) in mouse serum was measured by enzyme-linked immunosorbent assay using a human sIL2R immunoassay kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Pharmacokinetics analysis of BAY 1143572 in NOG mice
Three female NOG mice at 6 to 8 weeks of age (Taconic Biosciences, Hudson, NY) were treated by oral application of 12.5 mg/kg BAY 1143572, and then blood sampling was performed 0.5, 1, 3, and 7 hours after drug administration. Total plasma concentrations of BAY 1143572 were measured by liquid chromatography–tandem mass spectrometry (SCIEX, Framingham, MA). Unbound plasma levels were obtained by correcting total concentrations for protein binding from mouse plasma. Plasma protein binding was determined via the ultrafiltration method.
Statistical analysis
Differences between 2 groups were examined using Mann-Whitney U tests. Mouse survival analyses were carried out using the Kaplan-Meier method, and survival curves were compared using the log-rank test. All analyses were performed using SPSS Statistics 17.0 software (SPSS Inc, Chicago, IL). P < .05 was considered significant.
Results
BAY 1143572 inhibited growth of ATL-derived or HTLV-1–transformed lines
BAY 1143572 inhibited the proliferation of ATL-derived or HTLV-1–transformed lines in a dose-dependent manner (Figure 1A).
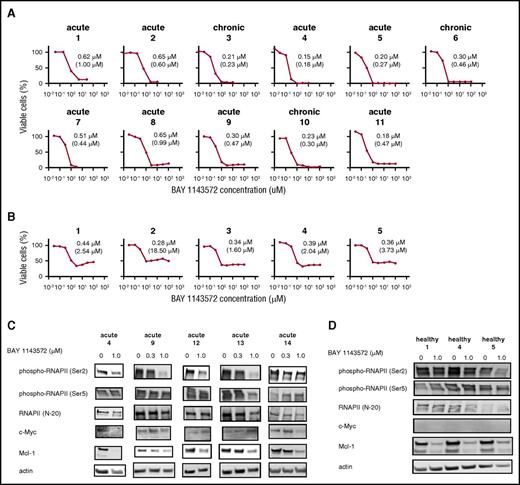
BAY 1143572 inhibits proliferation, induces apoptosis, and affects CDK9 signaling in ATL-derived or HTLV-1–transformed cell lines. (A) Viabilities of ATL-derived or HTLV-1–transformed cell lines in the presence of different concentrations of BAY 1143572 for 72 hours (ATN-1, MJ, MT-1, MT-2, TL-Om1, TL-Su, and MT-4) and 24 hours (S-YU) were assessed. S-YU cells were assessed in the presence of recombinant human IL-2 at a final concentration of 100 IU/mL. The IC50 value (the concentration of inhibitor where the response is reduced by half the maximal value) for each line is indicated in each panel. The absolute IC50 value (the concentration of an inhibitor that reduced cell survival to 50% of the untreated control value, with the highest observed viability [no inhibitor] defined as 100%, and the lowest viability defined as 0%) for each line is also indicated in parentheses below the IC50 value. (B) ATL-derived or HTLV-1–transformed cell lines were treated with various concentrations of BAY 1143572 for 72 hours. Apoptosis was then analyzed by Annexin V and propidium iodide (PI; nuclear) staining and flow cytometry. BAY 1143572 concentrations are indicated above the panels, and the percentage of cells in each quadrant is indicated in or beside each quadrant. (C) ATN-1, TL-Om1, MT-4, and MJ cell lines were treated with the indicated concentrations of BAY 1143572 for 5 hours, and western blotting was performed. Blots were probed with antibodies to phospho-RNAPII (Ser2 of the CTD [Ser2]), phospho-RNAPII (Ser5), RNAPII (N-20), c-Myc, Mcl-1, and HTLV-1 Tax. Actin was used as a loading control.
BAY 1143572 inhibits proliferation, induces apoptosis, and affects CDK9 signaling in ATL-derived or HTLV-1–transformed cell lines. (A) Viabilities of ATL-derived or HTLV-1–transformed cell lines in the presence of different concentrations of BAY 1143572 for 72 hours (ATN-1, MJ, MT-1, MT-2, TL-Om1, TL-Su, and MT-4) and 24 hours (S-YU) were assessed. S-YU cells were assessed in the presence of recombinant human IL-2 at a final concentration of 100 IU/mL. The IC50 value (the concentration of inhibitor where the response is reduced by half the maximal value) for each line is indicated in each panel. The absolute IC50 value (the concentration of an inhibitor that reduced cell survival to 50% of the untreated control value, with the highest observed viability [no inhibitor] defined as 100%, and the lowest viability defined as 0%) for each line is also indicated in parentheses below the IC50 value. (B) ATL-derived or HTLV-1–transformed cell lines were treated with various concentrations of BAY 1143572 for 72 hours. Apoptosis was then analyzed by Annexin V and propidium iodide (PI; nuclear) staining and flow cytometry. BAY 1143572 concentrations are indicated above the panels, and the percentage of cells in each quadrant is indicated in or beside each quadrant. (C) ATN-1, TL-Om1, MT-4, and MJ cell lines were treated with the indicated concentrations of BAY 1143572 for 5 hours, and western blotting was performed. Blots were probed with antibodies to phospho-RNAPII (Ser2 of the CTD [Ser2]), phospho-RNAPII (Ser5), RNAPII (N-20), c-Myc, Mcl-1, and HTLV-1 Tax. Actin was used as a loading control.
BAY 1143572 also inhibited the proliferation of T-ALL lines in a dose-dependent manner (Figure 2A).
BAY 1143572 inhibits proliferation, induces apoptosis, and affects CDK9 signaling in HTLV-1−human T-ALL cell lines. (A) Viabilities of T-ALL cell lines (Jurkat, CCRF-CEM, and TALL-1) in the presence of different concentrations of BAY 1143572 for 72 hours were assessed. The IC50 value for each line is indicated in each panel. The absolute IC50 value for each line is also indicated in parentheses below the IC50 value. (B) T-ALL cell lines were treated with various concentrations of BAY 1143572 for 72 hours. Apoptosis was then analyzed by Annexin V and PI (nuclear) staining and flow cytometry. BAY 1143572 concentrations are indicated above the panels, and the percentage of cells in each quadrant is indicated in or beside each quadrant. (C) Jurkat, CCRF-CEM, and TALL-1 cell lines were treated with the indicated concentrations of BAY 1143572 for 5 hours, protein extracts prepared, and western blotting performed. Blots were probed with antibodies to phospho-RNAPII (Ser2 of the CTD [Ser2]) phospho-RNAPII (Ser5), RNAPII (N-20), c-Myc, and Mcl-1. Actin was used as a loading control.
BAY 1143572 inhibits proliferation, induces apoptosis, and affects CDK9 signaling in HTLV-1−human T-ALL cell lines. (A) Viabilities of T-ALL cell lines (Jurkat, CCRF-CEM, and TALL-1) in the presence of different concentrations of BAY 1143572 for 72 hours were assessed. The IC50 value for each line is indicated in each panel. The absolute IC50 value for each line is also indicated in parentheses below the IC50 value. (B) T-ALL cell lines were treated with various concentrations of BAY 1143572 for 72 hours. Apoptosis was then analyzed by Annexin V and PI (nuclear) staining and flow cytometry. BAY 1143572 concentrations are indicated above the panels, and the percentage of cells in each quadrant is indicated in or beside each quadrant. (C) Jurkat, CCRF-CEM, and TALL-1 cell lines were treated with the indicated concentrations of BAY 1143572 for 5 hours, protein extracts prepared, and western blotting performed. Blots were probed with antibodies to phospho-RNAPII (Ser2 of the CTD [Ser2]) phospho-RNAPII (Ser5), RNAPII (N-20), c-Myc, and Mcl-1. Actin was used as a loading control.
BAY 1143572 induced apoptosis in ATL-derived or HTLV-1–transformed lines
BAY 1143572 induced apoptosis of ATL-derived or HTLV-1–transformed lines in a dose-dependent manner (Figure 1B).
BAY 1143572 also induced apoptosis of T-ALL lines in a dose-dependent manner (Figure 2B).
Effect of BAY 1143572 on CDK9 signaling in ATL-derived or HTLV-1–transformed lines
BAY 1143572 inhibited phosphorylation of RNAPII at a Ser2 site in a dose-dependent manner, with a strong effect seen in ATN-1, MT-4, and MJ cells, but a weak effect seen in TL-Om1 cells. BAY 1143572 did not inhibit phosphorylation of RNAPII at a Ser5 site, and it did not affect RNAPII protein levels in any of the cell lines tested. Subsequently, BAY 1143572 suppressed c-Myc and Mcl-1 protein levels in a dose-dependent manner in ATN-1, TL-Om1, MT-4 and MJ cells. On the other hand, BAY 1143572 did not suppress HTLV-1 Tax protein levels in MT-4 and MJ cells (Figure 1C). HBZ transcription levels varied among ATL-derived or HTLV-1–transformed lines tested in response to BAY 1143572 (supplemental Figure 1, available on the Blood Web site). Thus, BAY 1143572 inhibited the phosphorylation of RNAPII at a Ser2 site, resulting in the inhibition of c-Myc and Mcl-1 transcription in ATL-derived or HTLV-1–transformed lines.
BAY 1143572 also inhibited phosphorylation of RNAPII at a Ser2 site in a dose-dependent manner in all T-ALL lines tested. It subsequently suppressed c-Myc protein levels in CCRF-CEM and Jurkat cells, and also suppressed Mcl-1 protein levels in all T-ALL lines tested (Figure 2C).
BAY 1143572 inhibited growth of primary ATL cells isolated from patients
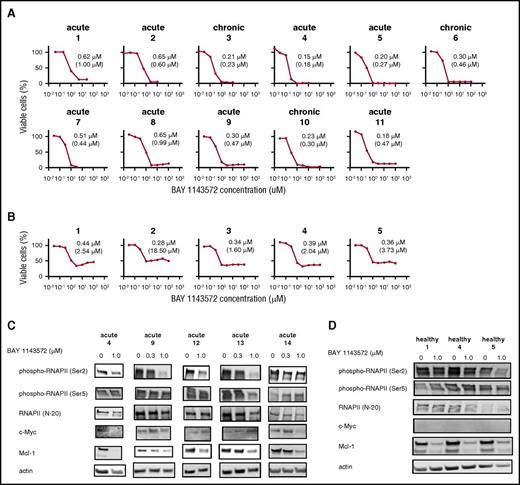
In a dose-dependent manner, BAY 1143572 also inhibited the proliferation of primary ATL cells isolated from patients (Figure 3A). In contrast with cell viability curves of primary ATL cells, those of CD4+ cells from healthy volunteers for BAY 1143572 showed an initial decrease, then a slight increase with increasing concentration of drug (Figure 3B). Absolute IC50 values for BAY 1143572 in primary ATL cells isolated from patients were significantly lower than those in CD4+ cells from healthy volunteers (P = .002).
BAY 1143572 inhibits proliferation in primary ATL cells from patients. (A) The viabilities of primary ATL cells from 11 individual patients, in the presence of recombinant human IL-2 at a final concentration of 100 IU/mL and with different concentrations of BAY 1143572 for 24 hours, were assessed. Primary ATL cells were isolated from PBMCs (nos. 1-8, 10, 11) or the affected lymph node (no. 9) of ATL patients. The clinical subtype of each ATL patient is indicated above each panel. The IC50 value for primary cells of each ATL patient is indicated in each panel and was determined by a cell proliferation assay. The absolute IC50 value for each primary ATL cell line is also indicated in parentheses below the IC50 value. (B) The viabilities of human CD4+ cells from PBMCs of 5 healthy volunteers, in the presence of recombinant human IL-2 at a final concentration of 100 IU/mL and with different concentrations of BAY 1143572 for 24 hours, were assessed. The IC50 value for cells isolated from each healthy volunteer is indicated in each panel and was determined by a cell proliferation assay. The absolute IC50 value for each CD4+ cell line is also indicated in parentheses below the IC50 value. (C) Primary ATL cells from 5 individual patients, and (D) CD4+ cells obtained from 3 healthy volunteers were treated with the indicated concentrations of BAY 1143572 for 12 hours, and western blotting was performed. Blots were probed with antibodies to phospho-RNAPII (Ser2), phospho-RNAPII (Ser5), RNAPII (N-20), c-Myc, and Mcl-1. Actin was used as a loading control.
BAY 1143572 inhibits proliferation in primary ATL cells from patients. (A) The viabilities of primary ATL cells from 11 individual patients, in the presence of recombinant human IL-2 at a final concentration of 100 IU/mL and with different concentrations of BAY 1143572 for 24 hours, were assessed. Primary ATL cells were isolated from PBMCs (nos. 1-8, 10, 11) or the affected lymph node (no. 9) of ATL patients. The clinical subtype of each ATL patient is indicated above each panel. The IC50 value for primary cells of each ATL patient is indicated in each panel and was determined by a cell proliferation assay. The absolute IC50 value for each primary ATL cell line is also indicated in parentheses below the IC50 value. (B) The viabilities of human CD4+ cells from PBMCs of 5 healthy volunteers, in the presence of recombinant human IL-2 at a final concentration of 100 IU/mL and with different concentrations of BAY 1143572 for 24 hours, were assessed. The IC50 value for cells isolated from each healthy volunteer is indicated in each panel and was determined by a cell proliferation assay. The absolute IC50 value for each CD4+ cell line is also indicated in parentheses below the IC50 value. (C) Primary ATL cells from 5 individual patients, and (D) CD4+ cells obtained from 3 healthy volunteers were treated with the indicated concentrations of BAY 1143572 for 12 hours, and western blotting was performed. Blots were probed with antibodies to phospho-RNAPII (Ser2), phospho-RNAPII (Ser5), RNAPII (N-20), c-Myc, and Mcl-1. Actin was used as a loading control.
Effect of BAY 1143572 on CDK9 signaling in primary ATL cells isolated from patients
BAY 1143572 inhibited phosphorylation of RNAPII at a Ser2 site in a dose-dependent manner, with a strong effect seen in primary ATL cells isolated from acute patients 9 and 13, and a moderate effect seen in cells isolated from acute patients 4 and 12; however, a weak effect was seen in cells isolated from acute patient 14. BAY 1143572 did not inhibit phosphorylation of RNAPII at a Ser5 site, and did not affect RNAPII protein levels in any of the primary ATL cells tested. Subsequently, BAY 1143572 suppressed c-Myc protein levels in primary ATL cells isolated from acute patients 4 and 14. BAY 1143572 also suppressed Mcl-1 protein levels in a dose-dependent manner in all primary ATL cells tested (Figure 3C).
In healthy volunteers, BAY 1143572 inhibited phosphorylation of RNAPII at a Ser2 site, with a strong effect seen in CD4+ cells from volunteer 5; however, a weak effect was observed in healthy volunteers 1 and 4. BAY 1143572 did not inhibit phosphorylation of RNAPII at a Ser5 site, and did not affect RNAPII protein levels in any of the CD4+ cells tested. Subsequently, BAY 1143572 suppressed Mcl-1 protein levels; however, c-Myc protein levels were not detected in CD4+ cells from all 3 healthy volunteers (Figure 3D).
Macroscopic and microscopic findings in mice with or without BAY 1143572 therapy
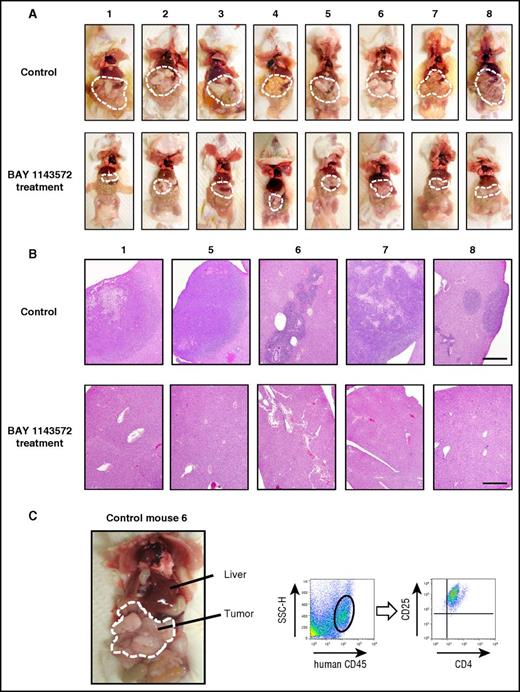
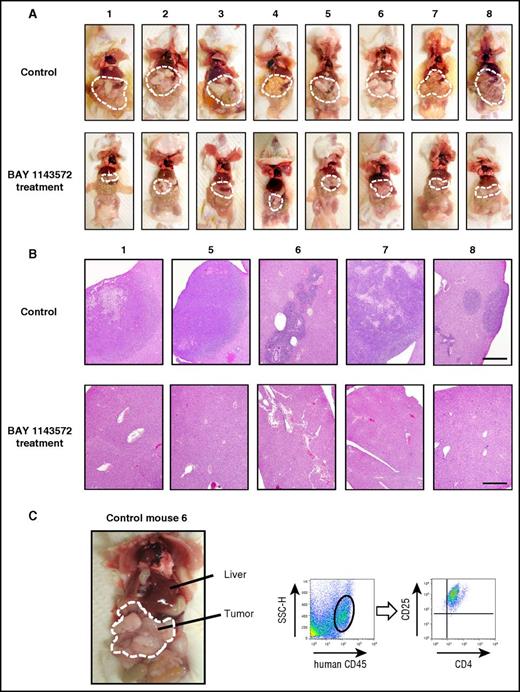
The appearance of control mice (treated with vehicle), or those treated with BAY 1143572, 25 days after ATL cell inoculation is shown in Figure 4A (top and bottom panels, respectively). Tumor masses are demarcated by thin white dashed lines, indicating that these were strikingly shrunken by BAY 1143572 treatment (Figure 4A). Photomicrographs of liver tissues from control mice numbers 1, 5, 6, 7, and 8, or those from BAY 1143572–treated mice numbers 1, 5, 6, 7, and 8, are shown in Figure 4B (top and bottom panels, respectively). Liver tissues from control mice were aggressively infiltrated by ATL cells and destroyed to various extents. In contrast, those from BAY 1143572–treated mice were histologically intact. Figure 4C shows i.p. masses and intestinal tracts adhering tightly to one another in control NOG mouse 6. Flow cytometry analysis of a tumor cell suspension derived from i.p. masses demonstrated that it consisted primarily of human cells expressing CD4 and CD25. Toxicity attributable to BAY 1143572 was not observed in any of the mice during the study period.
Characteristics of ATL cell–bearing NOG mice. (A) The macroscopic appearances of mice treated with vehicle (control; top panels) or BAY 1143572 (bottom panels). Tumor masses are demarcated by thin white dashed lines. (B) Photomicrographs of hematoxylin and eosin–stained liver sections from mice numbers 1, 5, 6, 7, and 8 treated by vehicle (control; top panels) or BAY 1143572 (bottom panels; scale bar, 500 μm). (C) The macroscopic appearance of ATL cell–bearing control mouse 6; i.p. tumor masses are demarcated by thin white dashed lines (left panel). Human CD45+ cells within masses were positive for CD4 and CD25, being consistent with ATL cells as determined by flow cytometry (the 2 right panels). SSC-H, side scatter height.
Characteristics of ATL cell–bearing NOG mice. (A) The macroscopic appearances of mice treated with vehicle (control; top panels) or BAY 1143572 (bottom panels). Tumor masses are demarcated by thin white dashed lines. (B) Photomicrographs of hematoxylin and eosin–stained liver sections from mice numbers 1, 5, 6, 7, and 8 treated by vehicle (control; top panels) or BAY 1143572 (bottom panels; scale bar, 500 μm). (C) The macroscopic appearance of ATL cell–bearing control mouse 6; i.p. tumor masses are demarcated by thin white dashed lines (left panel). Human CD45+ cells within masses were positive for CD4 and CD25, being consistent with ATL cells as determined by flow cytometry (the 2 right panels). SSC-H, side scatter height.
BAY 1143572 treatment reduced ATL cells in mouse liver
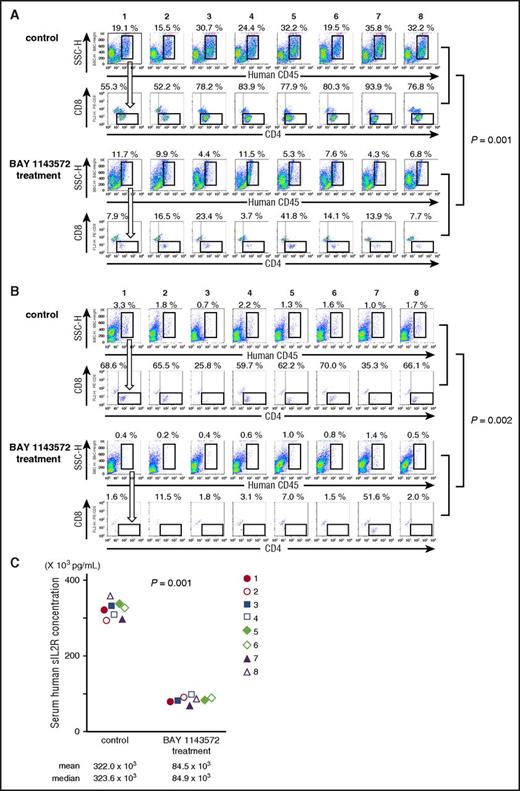
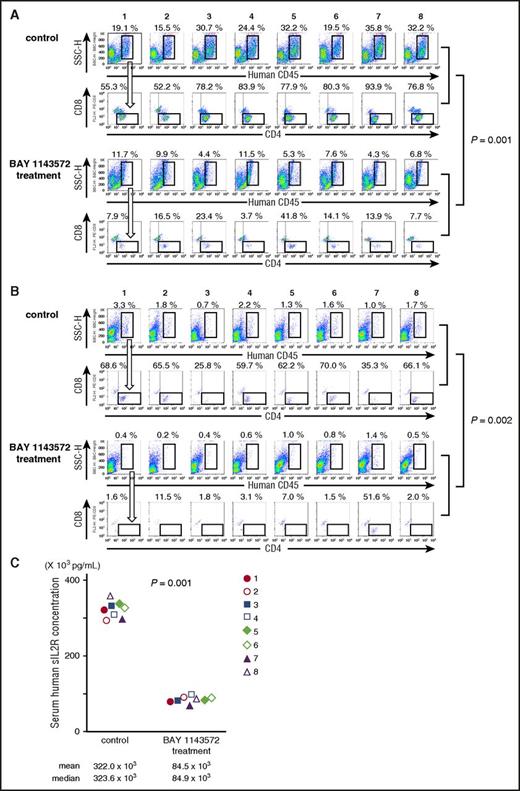
Twenty-five days after ATL cell inoculation, the percentage of ATL cells (human CD45+, CD4+, and CD8−) in a liver cell suspension from control NOG mouse 1 was 10.6% (ie, 19.1% [human CD45+ cells; Figure 5A topmost panel] × 55.3% [CD4+, but CD8− cells; Figure 5A second top panel] = 10.6%). In control NOG mice numbers 2, 3, 4, 5, 6, 7, and 8, and in BAY 1143572–treated NOG mice numbers 1, 2, 3, 4, 5, 6, 7, and 8, the percentages of ATL cells in liver cell suspensions, calculated in the same manner, were 8.1%, 24.0%, 20.5%, 25.1%, 15.7%, 33.6%, and 24.7%, and 0.9%, 1.6%, 1.0%, 0.4%, 2.2%, 1.1%, 0.6%, and 0.5%, respectively. Thus, BAY 1143572 treatment significantly decreased the percentage of ATL cells infiltrating the liver of mice inoculated with ATL cells (P = .001).
BAY 1143572 demonstrated significant therapeutic efficacies in ATL cell–bearing NOG mice. (A) Flow cytometric analyses of mouse liver cell suspensions are presented. The percentage of human CD45–expressing cells among mouse liver cell suspensions is indicated above each panel. Human CD45–expressing cells were plotted to show CD4 and CD8 expression. The percentage of ATL (CD4+ but CD8−) cells among human CD45+ cells is indicated above each panel. The top 2 lanes consist of panels showing control mice cell samples, and the bottom 2 lanes consist of panels showing BAY 1143572–treated mice cell samples. (B) Flow cytometric analyses of mice bone marrow cell suspensions. The percentage of human CD45–expressing cells among mouse bone marrow cell suspensions is indicated above each panel. Human CD45–expressing cells are plotted to show CD4 and CD8 expression. The percentage of ATL (CD4+ but CD8−) cells among human CD45+ cells is indicated above each panel. The top 2 lanes consist of panels showing control mice cell samples, and the bottom 2 lanes consist of panels showing BAY 1143572–treated mice cell samples. (C) Human sIL2R concentrations in serum from each of 8 ATL-bearing NOG mice. NOG mice treated with BAY 1143572 displayed significantly lower levels of sIL2R compared with control mice as determined by enzyme-linked immunosorbent assay (P = .001).
BAY 1143572 demonstrated significant therapeutic efficacies in ATL cell–bearing NOG mice. (A) Flow cytometric analyses of mouse liver cell suspensions are presented. The percentage of human CD45–expressing cells among mouse liver cell suspensions is indicated above each panel. Human CD45–expressing cells were plotted to show CD4 and CD8 expression. The percentage of ATL (CD4+ but CD8−) cells among human CD45+ cells is indicated above each panel. The top 2 lanes consist of panels showing control mice cell samples, and the bottom 2 lanes consist of panels showing BAY 1143572–treated mice cell samples. (B) Flow cytometric analyses of mice bone marrow cell suspensions. The percentage of human CD45–expressing cells among mouse bone marrow cell suspensions is indicated above each panel. Human CD45–expressing cells are plotted to show CD4 and CD8 expression. The percentage of ATL (CD4+ but CD8−) cells among human CD45+ cells is indicated above each panel. The top 2 lanes consist of panels showing control mice cell samples, and the bottom 2 lanes consist of panels showing BAY 1143572–treated mice cell samples. (C) Human sIL2R concentrations in serum from each of 8 ATL-bearing NOG mice. NOG mice treated with BAY 1143572 displayed significantly lower levels of sIL2R compared with control mice as determined by enzyme-linked immunosorbent assay (P = .001).
BAY 1143572 treatment reduced ATL cells in mouse bone marrow
The percentage of ATL cells (human CD45+, CD4+, and CD8−) in bone marrow cells of control NOG mouse 1 was 2.26% (ie, 3.3% [human CD45+ cells; Figure 5B topmost panel] × 68.6% [CD4+, but CD8− cells; Figure 5B second top panel] = 2.26%). In control NOG mice 2, 3, 4, 5, 6, 7, and 8, and in BAY 1143572–treated NOG mice 1, 2, 3, 4, 5, 6, 7, and 8, the percentages of ATL cells in bone marrow cell suspensions, calculated in the same manner, were 1.18%, 0.18%, 1.31%, 0.81%, 1.12%, 0.35%, and 1.12%, and 0.01%, 0.02%, 0.01%, 0.02%, 0.01%, 0.01%, 0.72%, and <0.01%, respectively. Thus, BAY 1143572 treatment significantly decreased the percentage of ATL cells infiltrating the bone marrow of mice inoculated with ATL cells (P = .002).
sIL2R concentrations in mice with or without BAY 1143572 therapy
The concentrations of human sIL2R in the serum of ATL cell–bearing control NOG mice 1 through 8 were 322.0 × 103 pg/mL, 323.6 × 103 pg/mL, and 293.0 × 103 pg/mL to 361.3 × 103 pg/mL (mean, median, and range, respectively), and those of BAY 1143572–treated NOG mice 1 through 8 were 84.5 × 103 pg/mL, 84.9 × 103 pg/mL, and 69.0 × 103 pg/mL to 97.4 × 103 pg/mL (mean, median, and range, respectively). Thus, BAY 1143572 significantly reduced serum levels of human sIL2R in mice inoculated with ATL cells (P = .001; Figure 5C).
BAY 1143572 induced a prolongation of survival time in ATL cell–bearing mice
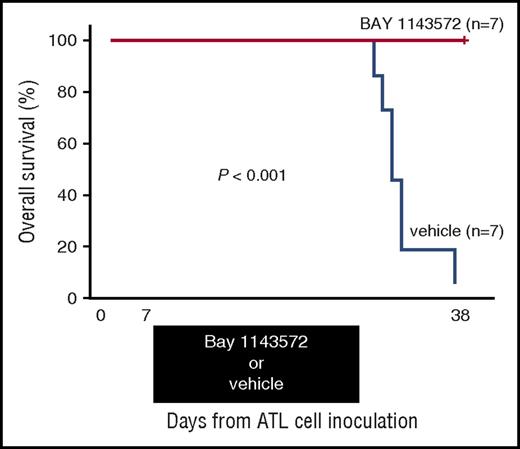
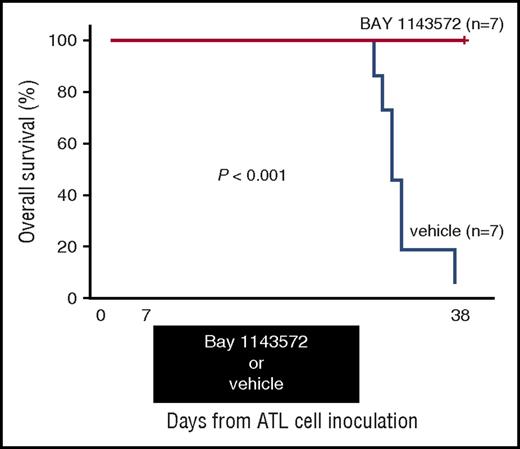
Thirty-eight days after ATL cell inoculation, BAY 1143572–treated mice were all alive (n = 7), but vehicle-treated control mice were all dead (n = 7). Toxicity attributable to BAY 1143572 was not observed in any of the mice during the study period. Thus, a BAY 1143572–treated mouse group inoculated with ATL cells displayed a significant prolongation of survival time compared with untreated controls (P < .001; Figure 6).
Kaplan-Meier survival curves of ATL cell–bearing NOG mice treated with BAY 1143572 or vehicle. Mice were treated by oral application of 12.5 mg/kg BAY 1143572 or vehicle (n = 7 for both), once daily for 21 days (7-27 days after ATL cell inoculations). The difference between the BAY 1143572 and vehicle-treated mice was statistically significant (P < .001).
Kaplan-Meier survival curves of ATL cell–bearing NOG mice treated with BAY 1143572 or vehicle. Mice were treated by oral application of 12.5 mg/kg BAY 1143572 or vehicle (n = 7 for both), once daily for 21 days (7-27 days after ATL cell inoculations). The difference between the BAY 1143572 and vehicle-treated mice was statistically significant (P < .001).
Effect of BAY 1143572 on CDK9 signaling in i.p. ATL masses of mice
BAY 1143572 inhibited phosphorylation of RNAPII at a Ser2 site that led to the suppression of c-Myc and Mcl-1 protein levels of i.p. ATL cells in treated mice. The inhibition of phosphorylation of RNAPII at a Ser5 site by BAY 1143572 was also observed in treated mice, but the extent was much weaker compared with the inhibition of RNAPII at a Ser2 site. RNAPII and actin protein amounts were also reduced in treated mice, particularly in mouse 1 (supplemental Figure 2). This observation was associated with the reduced viability of i.p. ATL cells in treated mice.
Pharmacokinetics of BAY 1143572 in NOG mice
The maximum unbound concentration of BAY 1143572 in mice was 1.37 μM (supplemental Table 1).
Discussion
In this study, we evaluated the therapeutic potential of BAY 1143572, a novel and highly selective CDK9/P-TEFb inhibitor, in ATL, which has a poor prognosis. We demonstrated that BAY 1143572 possessed significant antitumor activities against not only ATL-derived or HTLV-1–transformed lines, but also primary ATL cells in vitro. Additionally, we demonstrated that BAY 1143572 possessed significant antitumor activity in an ATL mouse model, in which tumor cells isolated from a patient survived and proliferated in a murine microenvironment. The present findings also revealed the importance of the CDK9-signaling system for the pathogenesis of ATL, suggesting that CDK9 signaling could be an ideal molecular target as a novel treatment of ATL.
First, we determined the anti-ATL mechanism of action of BAY 1143572 using ATL-derived or HTLV-1–transformed lines in vitro. We found that BAY 1143572 specifically inhibited the phosphorylation of RNAPII at Ser2, but not at Ser5. This subsequently led to the downregulation of downstream proteins such as c-Myc and Mcl-1. Contrary to other reports,20,21 BAY 1143572 did not clearly suppress HTLV-1 component genes such as Tax and, in addition, HBZ. Together with the potent effect of BAY 1143572 in HTLV-1− T-ALL lines, major antitumor mechanisms of this agent in ATL-derived or HTLV-1–transformed lines seemed not to be mediated by HTLV-1 component genes. Eventually, as a result, BAY 1143572 induced growth inhibition and apoptosis in ATL-derived or HTLV-1–transformed lines in a dose-dependent manner.
Almost identical results were observed in primary ATL cells. BAY 1143572 specifically inhibited the phosphorylation of RNAPII at Ser2, but not at Ser5, and this subsequently led to the downregulation of the downstream protein, Mcl-1. In contrast with observations in ATL-derived or HTLV-1–transformed lines, the expression levels of another downstream protein, c-Myc, were relatively low, and were weakly affected by BAY 1143572 in primary ATL cells. This seems to indicate that primary ATL cells do not aggressively proliferate in ex vivo culture conditions, in spite of the presence of recombinant human IL-2; this is almost consistent with our previous report.24 As a result, BAY 1143572 inhibited the survival of primary ATL cells in a dose-dependent manner. Exposure to 1.0 μM or greater concentrations of BAY 1143572 for 24 hours induced the death of almost all primary ATL cells. However, as with CD4+ cells from healthy volunteers, the cell viability curve after BAY 1143572 treatment demonstrated that exposure to 100 μM BAY 1143572 for 24 hours allowed nearly >30% of healthy CD4+ cells to survive. This strongly indicated that BAY 1143572 was less toxic for healthy CD4+ cells, from which ATL cells originated. In other words, CD4+ cells may acquire a deregulated CDK9-signaling system in the process of developing into ATL.
Because S-YU cells can only be maintained by serial transplantation in immunodeficient mice, but not by in vitro culture,26,27,33 the microenvironment is likely to be indispensable for their survival. Therefore, we consider that the present ATL model using S-YU cells better reflects the human ATL in vivo environment, compared with other mouse models using established ATL-derived or HTLV-1–transformed lines in vitro. In the present study, BAY 1143572 exhibited significant antitumor activity as demonstrated by the reduced degree of ATL cell infiltration into liver and bone marrow in ATL cell–bearing mice. Additionally, BAY 1143572 exhibited significant antitumor activity as demonstrated by reduced serum human sIL2R concentrations, which directly reflects the tumor burden of ATL cells34 in BAY 1143572–treated mice. Furthermore, we confirmed here that BAY 1143572 monotherapy significantly prolonged survival time in ATL cell–bearing mice. Importantly, the present study demonstrated that such potent in vivo antitumor activities of BAY 1143572 in ATL cell–bearing mice were actually mediated by the on-target effect of BAY 1143572, namely, the inhibition of CDK9 and subsequent inhibition of phosphorylation at Ser2 of the RNAPII CTD. We should also pay particular attention to the fact that the IC50 value of BAY 1143572 at 24 hours for the inoculated ATL cell line, S-YU, in the presence of recombinant human IL-2, was 1.47 μM. This in vitro IC50 value for BAY 1143572 was in the range of the measured maximum unbound concentration level of BAY 1143572 in NOG mice (1.37 μM); and, potent antitumor activities were observed in S-YU–bearing mice in the present study. Additionally, the in vitro IC50 values of BAY 1143572 in primary ATL cells (0.36 μM, 0.30 μM, and 0.15-0.65 μM, mean, median, and range, respectively) were much lower than those in S-YU cells. These findings collectively prompt us to consider that the antitumor effect of BAY 1143572 would be expected to be much more clearly observed in human ATL patients compared with that observed in ATL mice, as in the present study. Importantly, toxicity attributable to BAY 1143572 was not observed in any of the mice in the present in vivo studies. Therefore, these observations in ATL mice provide strong evidence that targeting CDK9 in humans with ATL could be a promising therapeutic approach.
To the best of our knowledge, this is the first report to evaluate the efficacy of CDK9-targeting therapy for peripheral T-cell neoplasms, including ATL. Several agents targeting CDK9, such as Flavopiridol,35-38 Dinaciclib,39,40 Seliciclib,41,42 SNS-032,15 and RGB-286338,43 have been used in the field of cancer therapy.44 However, treatment with such drugs continues to be unsuccessful and to involve many adverse events, possibly due to their lack of selectivity for CDK9 and their inhibition of other CDKs.45 In contrast, the present inhibitor, BAY 1143572, has high selectivity for CDK9/PTEFb,22 and demonstrated a promising antitumor effect for ATL, not only in in vitro experiments, but also in in vivo experiments. In conclusion, the present study demonstrated that CDK9/P-TEFb inhibition by BAY 1143572 showed strong potential as a novel treatment of patients with ATL. Further clinical evaluations of CDK9/P-TEFb–selective inhibitors in patients with ATL are warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chiori Fukuyama and Eriko Hosoba for excellent technical assistance, and Naomi Ochiai for expert secretarial assistance. The authors also thank Stuart Ince (Bayer US, LLC) for the kind contribution of obtaining BAY 1143572.
This work was supported by research funding from Bayer AG Pharmaceuticals Division to the Graduate School of Medical Sciences, Nagoya City University. This work was also supported by a grant-in-aid for scientific research (B) (no. 16H04713 [T.I.]), a grant-in-aid from the National Cancer Center Research and Development Fund (no. 26-A-4 [T.I. and S.I.]), and grants-in-aid from the Japan Agency for Medical Research and Development (nos. 16cm0106301h0001 [T.I.], 15ck0106159h0001 [T.I., A.I., and S. Kusumoto], and 15ck0106132h0002 [T.I., R.U., and S.I.]).
Authorship
Contribution: T.N., T.I., and R.U. designed the research; T.N., T.I., A.I., A.M., S. Kinoshita, S.S., H.T., T.Y., M.R., S. Kusumoto, H.K., K.I., Y.T., A.T.-K., H.I., P.L., and S.I. performed the experiments; A.S. had input into the design of the studies and, together with T.K., contributed to obtaining BAY 1143572; T.N., T.I., and R.U. analyzed and interpreted data; and all authors wrote and approved the manuscript.
Conflict-of-interest disclosure: T.I. obtained research funding from Kyowa Hakko Kirin Co, Ltd, Bayer Pharma AG, and J-Pharma Co, Ltd, and honoraria from Kyowa Hakko Kirin Co, Ltd, and Celgene KK. K.I. received honoraria from Janssen, Celgene KK, Takeda, Chugai Pharmaceutical Co, Ltd, Otsuka, Shire, FUJIFILM RI Pharma, Nippon Shinyaku, and Bristol-Myers Squibb. A.S. and P.L. are employees of Bayer AG Pharmaceuticals Division. T.K. is an employee of Bayer Yakuhin, Ltd. R.U. has a consultancy with Mundipharma KK, Ono Pharmaceutical Co, Ltd, and Terumo Co, Ltd; receives research funding from Kyowa Hakko Kirin Co, Ltd, Rikaken Co, Ltd, Medical & Biological Laboratories Co, Ltd, and Chugai Pharmaceutical Co, Ltd; and honoraria from Chugai Pharmaceutical Co, Ltd, Kyowa Hakko Kirin Co, Ltd, and Ono Pharmaceutical Co, Ltd. S.I. receives research funding from Ono Pharmaceutical Co, Ltd, Eli Lilly, Celgene KK, Janssen, Chugai Pharmaceutical Co, Ltd, Kyowa Hakko Kirin Co, Ltd, and Takeda, and honoraria from Janssen, Celgene KK, Ono Pharmaceutical Co, Ltd, Takeda, and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Takashi Ishida, Department of Hematology and Oncology, Graduate School of Medical Sciences, Nagoya City University, 1 Kawasumi, Mizuho-chou, Mizuho-ku, Nagoya, Aichi 467-8601, Japan; e-mail: itakashi@med.nagoya-cu.ac.jp.

![Figure 1. BAY 1143572 inhibits proliferation, induces apoptosis, and affects CDK9 signaling in ATL-derived or HTLV-1–transformed cell lines. (A) Viabilities of ATL-derived or HTLV-1–transformed cell lines in the presence of different concentrations of BAY 1143572 for 72 hours (ATN-1, MJ, MT-1, MT-2, TL-Om1, TL-Su, and MT-4) and 24 hours (S-YU) were assessed. S-YU cells were assessed in the presence of recombinant human IL-2 at a final concentration of 100 IU/mL. The IC50 value (the concentration of inhibitor where the response is reduced by half the maximal value) for each line is indicated in each panel. The absolute IC50 value (the concentration of an inhibitor that reduced cell survival to 50% of the untreated control value, with the highest observed viability [no inhibitor] defined as 100%, and the lowest viability defined as 0%) for each line is also indicated in parentheses below the IC50 value. (B) ATL-derived or HTLV-1–transformed cell lines were treated with various concentrations of BAY 1143572 for 72 hours. Apoptosis was then analyzed by Annexin V and propidium iodide (PI; nuclear) staining and flow cytometry. BAY 1143572 concentrations are indicated above the panels, and the percentage of cells in each quadrant is indicated in or beside each quadrant. (C) ATN-1, TL-Om1, MT-4, and MJ cell lines were treated with the indicated concentrations of BAY 1143572 for 5 hours, and western blotting was performed. Blots were probed with antibodies to phospho-RNAPII (Ser2 of the CTD [Ser2]), phospho-RNAPII (Ser5), RNAPII (N-20), c-Myc, Mcl-1, and HTLV-1 Tax. Actin was used as a loading control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/9/10.1182_blood-2016-09-741983/4/m_blood741983f1.jpeg?Expires=1767829812&Signature=Elb9LzW4pKOKrZ4oLwHZHRafyAliwyehYpGN44UyY4LTd0EwVQrmmhejPWwdky3tJii7b8a5DhafXWIdoacg0nxCqKbnbMZxE8rtXAdpQrK7QIp1kOcMCM64~zzoMYJEL5cdd1VkMo6JjECygdm-NPUTUektlELh3s8hKGIdAcXOPNwAB6L6klJ0E9b9NhBUJIS2lT943ZSIaNVTAlxEKEgozMm4x2MMnJXPY19wwwIh6aXhurYhG78sdh~3~kM74MdZ7b74-GsfYU1OUW54rTz5DwiUZn5dUn3NYeZNsTE8q-0s9JB1zxFrZ9fCFFeojwo9jSESFywMnawu9fAutw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. BAY 1143572 inhibits proliferation, induces apoptosis, and affects CDK9 signaling in HTLV-1−human T-ALL cell lines. (A) Viabilities of T-ALL cell lines (Jurkat, CCRF-CEM, and TALL-1) in the presence of different concentrations of BAY 1143572 for 72 hours were assessed. The IC50 value for each line is indicated in each panel. The absolute IC50 value for each line is also indicated in parentheses below the IC50 value. (B) T-ALL cell lines were treated with various concentrations of BAY 1143572 for 72 hours. Apoptosis was then analyzed by Annexin V and PI (nuclear) staining and flow cytometry. BAY 1143572 concentrations are indicated above the panels, and the percentage of cells in each quadrant is indicated in or beside each quadrant. (C) Jurkat, CCRF-CEM, and TALL-1 cell lines were treated with the indicated concentrations of BAY 1143572 for 5 hours, protein extracts prepared, and western blotting performed. Blots were probed with antibodies to phospho-RNAPII (Ser2 of the CTD [Ser2]) phospho-RNAPII (Ser5), RNAPII (N-20), c-Myc, and Mcl-1. Actin was used as a loading control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/9/10.1182_blood-2016-09-741983/4/m_blood741983f2.jpeg?Expires=1767829812&Signature=vAy9JVRTD7g81f1Qx6plwloqHzfFYOXmD5R~MPjHpyw20inht98MF4q2TjWF6qhDpf95hx1niJ2lzmrCPKiDiZCMXPbEwzshZUDOeFRxZ3ZeBd8lcsEN05Fsv7Z7iJxIjdNoX8aO7OJdI3KQoCtVLoRr7KyIZlAmoy5o2OZkAxui7z~Nh1Na1nNNH6i3wUEE1vUodTk7ck3wQXFwHIHfr7tQBGZGp7Xhz40B-FUmHU09lBENg~wdbZOD1nJ7J1PrD8nxciQ1rlD1~M2sZVkbJzCFMyw3ynZivDAqp~InFpx10GMK8eRX4CFtb~Drv657FOntb7au-MtTHy1KW6rIHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. BAY 1143572 inhibits proliferation, induces apoptosis, and affects CDK9 signaling in ATL-derived or HTLV-1–transformed cell lines. (A) Viabilities of ATL-derived or HTLV-1–transformed cell lines in the presence of different concentrations of BAY 1143572 for 72 hours (ATN-1, MJ, MT-1, MT-2, TL-Om1, TL-Su, and MT-4) and 24 hours (S-YU) were assessed. S-YU cells were assessed in the presence of recombinant human IL-2 at a final concentration of 100 IU/mL. The IC50 value (the concentration of inhibitor where the response is reduced by half the maximal value) for each line is indicated in each panel. The absolute IC50 value (the concentration of an inhibitor that reduced cell survival to 50% of the untreated control value, with the highest observed viability [no inhibitor] defined as 100%, and the lowest viability defined as 0%) for each line is also indicated in parentheses below the IC50 value. (B) ATL-derived or HTLV-1–transformed cell lines were treated with various concentrations of BAY 1143572 for 72 hours. Apoptosis was then analyzed by Annexin V and propidium iodide (PI; nuclear) staining and flow cytometry. BAY 1143572 concentrations are indicated above the panels, and the percentage of cells in each quadrant is indicated in or beside each quadrant. (C) ATN-1, TL-Om1, MT-4, and MJ cell lines were treated with the indicated concentrations of BAY 1143572 for 5 hours, and western blotting was performed. Blots were probed with antibodies to phospho-RNAPII (Ser2 of the CTD [Ser2]), phospho-RNAPII (Ser5), RNAPII (N-20), c-Myc, Mcl-1, and HTLV-1 Tax. Actin was used as a loading control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/9/10.1182_blood-2016-09-741983/4/m_blood741983f1.jpeg?Expires=1767829813&Signature=P42A8d06VuQE6971xSe-Pg5RkTsQmX4jzHIgdzYTJDxMYsr9dXjFFGM520yeQAX23l5eL69xddDtW0itjUL1bf9CnxRIA1LvTRqipxEDYwWFjV~dFuV17FSc0-GnRAW1PO8LR4I80EpA9npDmloJB6BsUwNJCbYYaiCkWt3Gw2o-laHEhp73pkRZaXva2U9XT0WY5abvnvnOvut6CF3aGSNAmZQ0MQsdBcWE7kayj9CIWm13pa0ekm0FDVaK33PTBZbEFx0DiME9lr96x3DhUn1DijiYiIGJ8HX0waqxnfPNiIVEkb8GvGjMxG8wNp5BHqPj9g6Cg6InwydeQZzgOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. BAY 1143572 inhibits proliferation, induces apoptosis, and affects CDK9 signaling in HTLV-1−human T-ALL cell lines. (A) Viabilities of T-ALL cell lines (Jurkat, CCRF-CEM, and TALL-1) in the presence of different concentrations of BAY 1143572 for 72 hours were assessed. The IC50 value for each line is indicated in each panel. The absolute IC50 value for each line is also indicated in parentheses below the IC50 value. (B) T-ALL cell lines were treated with various concentrations of BAY 1143572 for 72 hours. Apoptosis was then analyzed by Annexin V and PI (nuclear) staining and flow cytometry. BAY 1143572 concentrations are indicated above the panels, and the percentage of cells in each quadrant is indicated in or beside each quadrant. (C) Jurkat, CCRF-CEM, and TALL-1 cell lines were treated with the indicated concentrations of BAY 1143572 for 5 hours, protein extracts prepared, and western blotting performed. Blots were probed with antibodies to phospho-RNAPII (Ser2 of the CTD [Ser2]) phospho-RNAPII (Ser5), RNAPII (N-20), c-Myc, and Mcl-1. Actin was used as a loading control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/9/10.1182_blood-2016-09-741983/4/m_blood741983f2.jpeg?Expires=1767829813&Signature=kELH6fgSmeZQEEquefBQH2-HqTci1QDfoesZ1wZbeR0UThDaHCEggT6WqiWbKudlTJoopY~xw-yNu6VBfjVvn2Z6LqS492bEwkuIViQMYY5uvEpSAWnX4iS7NWgPe9yQ96Io0gYN1oyWxj~2PpfOow1dev4JLsFn8~T3x89ZwQTB7mo-rfUybbZLj1mo8HPLhPDh~btF63dq0UkZXGZ4qIGtDqWiRzdNRQDqHRgDStplpjw4U5O2p-AnT94maFUYqqSK8KEd0ivkyRe7YfggbhEmq0BpeB0fdQob-6z0ENzUPTu5CD1Klq4-~t1p1SlB3CGi0DQmtG8egOku6e~C2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)