In this issue of Blood, Narita et al demonstrate that inhibition of cyclin-dependent kinase 9 (CDK9) signaling with the novel agent BAY 1143572 abrogates proliferation and induces apoptosis of adult T-cell leukemia/lymphoma (ATL) cells in cell lines, patient samples, and in xenografts in NOG mice.1 This makes CDK9 a potentially promising therapeutic target in ATL, a deadly disease.
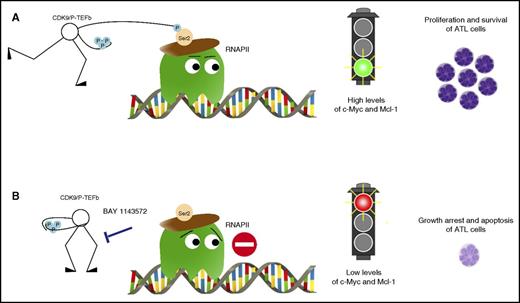
Inhibition of CDK9 signaling with the novel agent BAY 1143572 abrogates proliferation and induces apoptosis of ATL cells. (A) CDK9 phosphorylates RNA polymerase II (RNAPII) at serine 2 resulting in high levels of c-Myc and Mcl-1 and leading to proliferation and survival of ATL cells. (B) BAY 1143572 specifically inhibited CDK9-mediated phosphorylation at serine 2 of RNAPII resulting in decreased levels of c-Myc and Mcl-1, thus leading to growth arrest and apoptosis of ATL cells.
Inhibition of CDK9 signaling with the novel agent BAY 1143572 abrogates proliferation and induces apoptosis of ATL cells. (A) CDK9 phosphorylates RNA polymerase II (RNAPII) at serine 2 resulting in high levels of c-Myc and Mcl-1 and leading to proliferation and survival of ATL cells. (B) BAY 1143572 specifically inhibited CDK9-mediated phosphorylation at serine 2 of RNAPII resulting in decreased levels of c-Myc and Mcl-1, thus leading to growth arrest and apoptosis of ATL cells.
ATL is an aggressive proliferation of mature activated T cells transformed by human T-cell lymphotropic virus type-1 (HTLV-1) infection.2 Despite more than 35 years of intensive research, prognosis for patients with ATL remains dismal, with most patients dying within 1 year.3 This poor prognosis is the result of the intrinsic chemotherapy resistance of ATL cells and severe immune deficiency. The antiviral combination of zidovudine and interferon-α prolongs survival of patients with chronic and smoldering ATL subtypes and of a fraction of acute ATL patients whose cells contain wild-type P53.4 Allogeneic stem cell transplantation results in prolonged survival of approximately one-third of patients, but unfortunately, only a small percentage of ATL patients can receive a transplant.5 New promising agents include arsenic trioxide, lenalidomide, and mogamulizumab.3 Nevertheless, there is an unmet need for improved therapy for most ATL patients, stressing the need for novel targeted therapies.
CDK9 is a subunit of the positive transcription elongation factor b (P-TEFb) complex.6 It regulates the elongation step during gene transcription by phosphorylating the C-terminal domain of RNA polymerase II. Deregulation of CDK9/P-TEFb signaling has been found to play an important role in the development and/or maintenance of the malignant cell phenotype,7,8 making CDK9 an attractive therapeutic target. The novel and selective CDK9 inhibitor BAY 1143572 is currently being tested in phase 1 studies in patients with advanced solid tumors and acute leukemia.9
Narita et al investigated the efficacy of BAY 1143572 in ATL. They used multiple in vitro models that included ATL-derived or HTLV-I–transformed cell lines, primary leukemic cells obtained from ATL patients, and in vivo xenografts of patient-derived ATL cells in immunocompromised NOG mice. They demonstrate that BAY 1143572 specifically inhibited CDK9-mediated phosphorylation at serine 2 of RNA polymerase II, resulting in decreased levels of c-Myc and Mcl-1, thus leading to growth arrest and apoptosis of ATL cells (see figure). These effects were observed both in vitro and in vivo. Notably, a single oral administration of BAY 1143572 on a daily basis resulted in a striking decrease in ATL tumor infiltration in the liver and bone marrow of treated mice. This effect was also associated with decreased human soluble interleukin-2 receptor levels in the serum (indicative of the ATL tumor bulk) and resulted in a significant prolongation of mouse survival (no death in animals treated with BAY 1143572 vs 100% death in the untreated controls).
These findings reveal the importance of the CDK9 signaling in ATL pathogenesis and make CDK9 signaling an attractive and promising molecular and therapeutic target in ATL. The current report suggests that BAY 1143572 and other CDK9 inhibitors warrant testing in ATL patients, either alone or in combination with other effective therapies such as the combination of zidovudine, interferon-α, and arsenic trioxide.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal