In this issue of Blood, Newberry et al provide evidence that clonal evolution in patients with myelofibrosis (MF) who are receiving therapy with ruxolitinib correlates with discontinuation of treatment, mainly because of loss of response or progression, and also predicts shorter survival thereafter.1
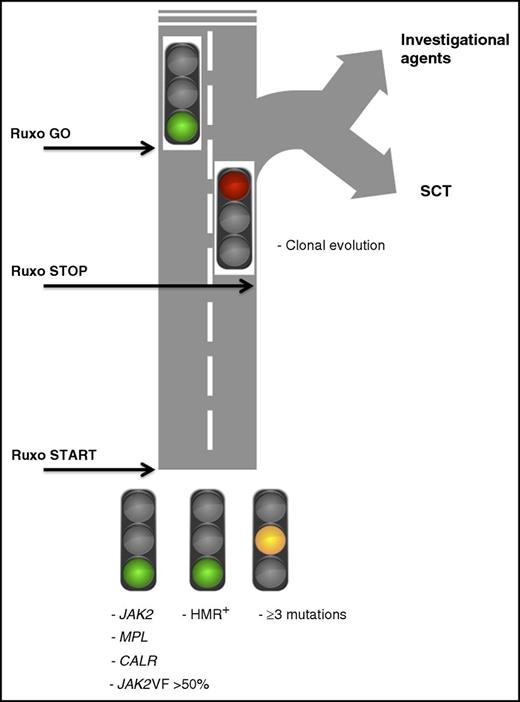
A color card depicting how results of a mutation profile in patients with MF might be used to make decisions about ruxolitinib (Ruxo) therapy at baseline and during treatment. JAK2VF >50%, presence of JAK2V617F mutation with an allelic burden >50%; HMR+, presence of high-molecular-risk mutations (ASXL1, EZH2, SRSF2, IDH1, IDH2); SCT, stem cell transplantation.
A color card depicting how results of a mutation profile in patients with MF might be used to make decisions about ruxolitinib (Ruxo) therapy at baseline and during treatment. JAK2VF >50%, presence of JAK2V617F mutation with an allelic burden >50%; HMR+, presence of high-molecular-risk mutations (ASXL1, EZH2, SRSF2, IDH1, IDH2); SCT, stem cell transplantation.
The JAK1 and JAK2 inhibitor ruxolitinib has become the standard of care for most patients with MF; it provides rapid and sustained reduction of splenomegaly and improvement in symptoms and quality of life, possibly resulting in prolongation of survival.2 Long-term follow-up showed that about half the patients enrolled in the COMFORT-I and COMFORT-II studies discontinued treatment by 3 years, largely because of loss of response and/or disease progression. The mechanisms of resistance and/or loss of response to ruxolitinib have not yet been deciphered. In cell lines, acquisition of mutations in the predicted ruxolitinib-binding region conferred resistance to JAK inhibitors,3 but such events have not been documented in patients. The phenomenon of persistence to a JAK2 inhibitor (ie, the fact that JAK2V617F-mutated cells survive despite chronic JAK inhibition) was ascribed to heterodimerization between activated JAK2 and other members of the JAK family and was shown to occur in cell lines, murine models, and patients treated with JAK2 inhibitors; however, the clinical relevance of such observations remains unsettled.4
Mutations in JAK2, MPL, and CALR are all involved in the abnormal activation of JAK/STAT signaling. This is not surprising (and it is good for patients) that ruxolitinib is efficacious irrespective of the underlying driver mutation. A JAK2V617F allele burden >50% predicts for better response to ruxolitinib,5 although this observation requires confirmation. In the COMFORT-II study,6 selected nondriver mutations included in the high-molecular-risk (HMR) category (ie, mutations in ASXL1, EZH2, SRSF2, IDH1, and IDH2)7 had no impact on the likelihood of obtaining early splenic response and symptomatic improvement. However, in the same patient cohort as in the Newberry et al report, it was shown by using an enlarged mutation panel that those patients with 3 or more mutations at study entry were more likely to discontinue treatment early and had lower odds of achieving spleen response.8 These latter findings suggest that patients with higher mutation complexity are less likely to obtain sustained responses to ruxolitinib, in line with findings that the number of mutations is independently associated with shorter survival and increased risk of leukemic transformation.9,10 When deciding on initial treatment using ruxolitinib, the above molecular information represents green and yellow lights for starting the treatment (see figure).
The study by Newberry et al has identified variables that are associated with and potentially predict for shorter duration of response to ruxolitinib and provides data on prognosis in patients who discontinued treatment. The core study population consisted of 56 patients with MF enrolled in a phase 1/2 study who discontinued ruxolitinib; in 42 patients, paired samples were available for molecular analysis and were used for target resequencing with a panel of 28 myeloid neoplasm–associated mutated genes (mutations in SRSF2 that are relatively frequent and harbor negative prognostic significance7 were not included in the panel). At the time of treatment discontinuation, hemoglobin and platelet count were reduced compared with baseline, whereas the proportion of patients with transfusion dependence increased from 29% to 43%. These findings are compatible with disease progression and with hematologic toxicity resulting from ruxolitinib. Clonal evolution, defined by the acquisition of 1 or more mutations at discontinuation, was reported in 35% of patients with available paired samples (n = 62); mutations mostly occurred in ASXL1 (68%), followed by TET2, EZH2, and TP53. Of importance, survival after discontinuation was remarkably shorter for the patients with clonal evolution (6 months) compared with all others (16 months). A platelet count of <260 × 109/L at baseline or <100 × 109/L at discontinuation also predicted for shortened survival, whereas acquiring transfusion dependency during ruxolitinib therapy turned out to be the only clinical variable correlated with clonal evolution. The proportion of patients with a complex karyotype at discontinuation rose from 13% at baseline to 25%, but this was independent of clonal evolution. Therefore, acquisition of new mutations during therapy with ruxolitinib would turn the traffic light to red, indicating the need to switch directions (see figure).
Recognizing the clinical patterns and/or the biomarkers that might predict clinical response in patients with MF receiving ruxolitinib represents a major goal for the appropriate and cost-effective use of this medication. Because survival after discontinuation was significantly shorter among patients who presented evidence of clonal evolution, one might anticipate that the early detection of newly acquired mutations represents a decision node (see figure) in favor of alternative therapies or stem cell transplantation when feasible. Unfortunately, the complexity and cost of genetic tests, uncertainties about when to obtain them during ruxolitinib treatment, and the fact that only one-third of the patients who discontinued ruxolitinib in the study actually had evidence of clonal progression, mark the boundary between theory and clinical practice. At present, such an approach is not feasible in patients treated with ruxolitinib outside clinical studies.
One concerning aspect that could not be dissected in the Newberry et al study is whether the acquisition of additional mutations results from the selective pressure exerted by ruxolitinib on the founding clone, thus facilitating the overgrowth of preexisting mutated clones initially below the detection threshold. Or does it represent true acquisition of novel mutations as a result of clonal genetic instability exacerbated by ruxolitinib? Or is it intrinsically related to the progression of disease and has nothing to do with ruxolitinib? Comparative studies of patients receiving no therapy or conventional therapy vs ruxolitinib might provide useful insights in this regard.
One key take-home message for clinicians from the Newberry et al study is that patients who discontinue ruxolitinib experience dismally short survival. Novel drugs for the treatment of these patients are urgently needed; unfortunately, there are none on the horizon.
Conflict-of-interest disclosure: A.M.V. received honoraria from Novartis for participating in advisory boards and giving lectures. P.G. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal