Key Points
Coro1A is identified as a novel regulator of β2 integrins (CD11/CD18).
Coro1A controls PMN adhesion and postadhesion events in innate immunity.
Abstract
Trafficking of polymorphonuclear neutrophils (PMNs) during inflammation critically depends on the β2 integrins lymphocyte function–associated antigen 1 (LFA-1) (CD11a/CD18) and macrophage-1 antigen (CD11b/CD18). Here, we identify coronin 1A (Coro1A) as a novel regulator of β2 integrins that interacts with the cytoplasmic tail of CD18 and is crucial for induction of PMN adhesion and postadhesion events, including adhesion strengthening, spreading, and migration under flow conditions. Transition of PMN rolling to firm adhesion critically depends on Coro1A by regulating the accumulation of high-affinity LFA-1 in focal zones of adherent cells. Defective integrin affinity regulation in the genetic absence of Coro1A impairs leukocyte adhesion and extravasation in inflamed cremaster muscle venules in comparison with control animals. In a Helicobacter pylori mouse infection model, PMN infiltration into the gastric mucosa is dramatically reduced in Coro1A−/− mice, resulting in an attenuated gastric inflammation. Thus, Coro1A represents an important novel player in integrin biology, with key functions in PMN trafficking during innate immunity.
Introduction
Severe combined immunodeficiency 8 (IMD8; OMIM 615401) is caused by homozygous or compound heterozygous mutations in the human coronin 1A (Coro1A) gene, leading to the loss of Coro1A protein expression.1-3 The genetic absence of Coro1A compromises development, homeostasis, and function of T cells caused by defective T-cell receptor (TCR) signaling.2-5 Accordingly, IMD8 patients suffer from a decreased number of circulating CD4+/CD8+ T cells and impaired T-cell function, resulting in sustained viral infections and the development of Epstein-Barr virus–associated lymphoproliferative disease.2,3 Moreover, the clinical presentation of IMD8 patients is characterized by recurrent bacterial infections,2-4 pointing toward a role of Coro1A in innate immunity.
The innate immune response relies on trafficking of polymorphonuclear neutrophils (PMNs) to sites of lesion, following a multistep recruitment process. Initiated via selectin-mediated capturing of free-flowing leukocytes by the inflamed endothelium and subsequent leukocyte rolling, slow leukocyte rolling allows the induction of firm PMN adhesion.6 This process critically depends on 2 leukocyte adhesion molecules of the β2 integrin family (CD11/CD18), namely lymphocyte function–associated antigen 1 (LFA-1) (CD11a/CD18) and macrophage-1 antigen (Mac-1) (CD11b/CD18). Whereas the transition of LFA-1 from its inactive conformation with low ligand binding affinity to its intermediate affinity state is required for slow leukocyte rolling,7 firm adhesion under flow conditions and subsequent postadhesion events depend on LFA-1 in its high-affinity conformation, a state that crucially involves cytoskeletal rearrangements.8 These cytoskeletal reorganization processes, which are orchestrated by integrin signaling upon ligand binding, are not only critical for LFA-1-mediated induction of PMN adhesion under flow but also important for LFA-1- and Mac-1-dependent postarrest functions of PMNs, including adhesion strengthening, spreading, intraluminal crawling, transendothelial diapedesis, and abluminal crawling.9-12
Coro1A belongs to a family of evolutionary conserved actin-binding proteins that regulate actin cytoskeleton-dependent processes such as cytokinesis, cell polarization, migration, and phagocytosis.13-17 In the mammalian system, Coro1A is predominantly expressed in leukocytes18 and plays an important role, for example, in Ca2+ signaling in macrophages,19 TCR signaling, and lymphocyte trafficking.5,20,21 Given that signaling of classical immunoreceptors and β2 integrins is similar,22 we explored the role of Coro1A for trafficking of PMNs in innate immunity. Here, Coro1A was identified as a novel regulator of β2 integrins (CD11/CD18), which interacts with the cytoplasmic tail of CD18. Moreover, we unraveled the biological significance of Coro1A for PMN trafficking, that is, induction of adhesion, adhesion strengthening, spreading, and migration. Notably, in a Helicobacter pylori (H pylori) mouse infection model, PMN trafficking and gastric infiltration of PMNs were substantially impaired in the genetic absence of Coro1A. This is of specific interest because H pylori infection affects more than 50% of the human population worldwide.23 Detailed live-cell imaging analysis revealed that compromised PMN trafficking was due to defective LFA-1 affinity regulation, identifying Coro1A as a novel player in integrin biology during host defense and inflammation.
Methods
Mice
Mice carrying the Coro1Atm1Jpie allele (Coro1A−/−) were kindly provided by Jean Pieters (University of Basel, Basel, Switzerland)19 and maintained on a C57BL/6 genetic background. All animal experiments were conducted in accordance with German federal animal protection laws and were approved by the Bavarian Government (Regierung von Oberbayern, Munich, Germany). PMN isolation and culture as well as flow cytometry analyses are described in the supplemental Methods (available on the Blood Web site).
Microscopy
Analysis of subcellular distribution of Coro1A, CD18, F-actin, or CD31 in vitro and in vivo was performed using confocal laser scanning microscopy (CLSM), confocal superresolution microscopy (CSRM), or stimulated emission depletion (STED) nanoscopy. For single-cell analysis in vitro, dHL-60-Coro1A–enhanced green fluorescent protein (EGFP) cells or Coro1A+/+ PMNs were either left untreated or stimulated with 100 nM of formyl-methionyl-leucyl-phenylalanine (fMLP) (dHL-60-Coro1A-EGFP), 10 µM fMLP (Coro1A+/+ PMNs), 1 mM of Mn2+ (dHL-60-Coro1A-EGFP) or 3 mM of Mn2+ (Coro1A+/+ PMNs) upon exposure to immobilized fibrinogen (250 µg/mL for dHL-60-Coro1A-EGFP; 50 µg/mL for Coro1A+/+ PMNs). After fixation, permeabilization, and blocking, immunostaining was performed as described in the supplemental Methods. CSRM and STED nanoscopy were conducted using a Leica TCS SP8 STED microscope equipped with a ×100/1.4-NA oil immersion objective (Leica, Germany). Images were analyzed offline with LAS X software (Leica, Germany) and ImageJ (National Institutes of Health [NIH], Bethesda, MD). For in vivo analysis, cremaster muscle whole mounts of Coro1A+/+ mice were prepared, as has been described, upon trauma- or recombinant murine tumor necrosis factor alpha (rmTNF-α)-induced inflammation.24 Prior to preparation, phycoerythrin-labeled rat anti-Ly6-G monoclonal antibody (mAb, clone 1A8; Biolegend, San Diego, CA; 10 μg per animal) was injected intravenously. After fixation, permeabilization, and blocking, cremaster muscle tissue was stained using the antibodies described in the supplemental Methods. Samples were mounted in PermaFluor (ThermoFisher Scientific, Germany) and CLSM was conducted using a Leica TCS SP5 microscope with a ×63/1.4-NA oil immersion objective (Leica, Germany). Images were analyzed offline using LAS AF software (Leica, Germany) and ImageJ (NIH). After surgical preparation of fMLP- or rmTNF-α-treated cremaster muscle of Coro1A+/+ and Coro1A−/− mice, leukocyte trafficking was investigated using classical and 2-photon intravital microscopy, as is described in detail in the supplemental Methods. Spinning-disk confocal microscopy was used for analysis of LFA-1 affinity on the cell surface of dHL60-Coro1A-EGFP cells and for analysis of the subcellular localization of Coro1A and F-actin, as is described in the supplemental Methods.
Adhesion assays
Static adhesion of Coro1A+/+ and Coro1A−/− PMNs was analyzed in triplicates using 96-well microtiter plates, as has been described.25 Briefly, PMNs (105 cells/sample) were plated on immobilized murine fibrinogen (Innovative Research, Novi, MI; 50 µg/mL) or rmICAM-1 (Stemcell, Germany; 12.5 µg/mL). Upon stimulation with 10 µM of fMLP (Sigma Aldrich, Germany), 5 µg/mL rmCXCL1 (Peprotech, Germany), or 3 mM of Mn2+ at 37°C for 10 minutes, samples were washed with phosphate-buffered saline, and attached cells were fixed with 1% glutaraldehyde. Absorbance of 0.1% Crystal Violet (Sigma Aldrich, Germany) was measured with a microplate reader (PowerWave HT, Biotek, Winooski, VT). To study induction of adhesion under flow, we perfused Coro1A+/+ and Coro1A−/− PMNs (7.5 × 105 per mL) through µ-slides VI0.1 (Ibidi, Germany) coated with 10 µg/mL of rmP-selectin-Fc (R&D Systems, Minneapolis, MN), 12.5 µg/mL of rmICAM-1, and 5 µg/mL of rmCXCL1 at constant shear stress (1 dyne/cm2) for 9 minutes. Time-lapse videos were recorded from 18 different fields of view with an Axiovert 200M microscope equipped with a Plan-Apochromat ×20/0.75-NA objective, AxioCam HR digital camera, and a temperature-controlled environmental chamber (Zeiss, Germany). The number of adherent PMNs and PMNs interacting with the surface were counted offline using ImageJ software (NIH). Relative adhesion was quantified by dividing the accumulated number of adherent cells by the accumulated number of all interacting cells (100%). Inhibition of LFA-1 or Mac-1 was performed using function blocking rat anti-mouse CD11a mAb (clone M17/4, BD Biosciences, San Jose, CA) or rat anti-mouse CD11b mAb (clone M1/70, BD Biosciences). Nonbinding rat IgG2a or rat IgG2b mAb (BD Biosciences) was used as isotype control. Adhesion strengthening, spreading, polarization, and migration assays are described in the supplemental Methods.
H pylori–induced gastritis
Coro1A+/+ and Coro1A−/− mice at ages between 8 and 10 weeks were infected orogastrically twice on 2 subsequent days with a dose of 109 bacteria of the H pylori strain X47 wild-type. Age-matched control animals were inoculated with sterile Brucella Broth (BB) medium (Oxoid, UK). After 6 weeks, mice were sacrificed, and stomachs were opened along the greater curvature. Whole stomachs were divided into 2 equal halves. One half was homogenized in BB medium, and appropriate dilutions were spread on selective serum plates (Oxoid, UK), supplemented with 80 mL/L of horse serum (PAA Laboratories, Cölbe, Germany), 10 mg/L of vancomycin, 5 mg/L of trimethoprim, 1 mg/L of nystatin, and 250 mg/L of streptomycin (all from Sigma Aldrich, Germany) and incubated under microaerophilic conditions (85% N2, 10% CO2, 5% O2) at 37°C for up to 5 days. The number of colony-forming units (CFU) was calculated per gram of gastric tissue. The other half of the stomach was formalin-fixed for histopathological analysis. Upon paraffin embedding, longitudinal sections of the stomach were stained by hematoxylin and eosin. Gastritis scores and intensity of inflammation (scale 0–3) were analyzed, as has been described.26,27 The numbers of emigrated PMNs were counted in the area of maximum inflammation analyzed through high power field magnification.
Statistics
Statistical significance (*P < .05; **P < .001) was determined by unpaired Student’s t test, by Mann-Whitney rank sum test, or by 2-way analysis of variance by using SigmaStat 3.5 (Systat Software, Germany) or GraphPad Prism 6 (GraphPad Software, USA). Data shown represent means ± standard deviation or means ± standard error of the mean, as are indicated in the figures.
Results
Role of Coro1A in integrin biology
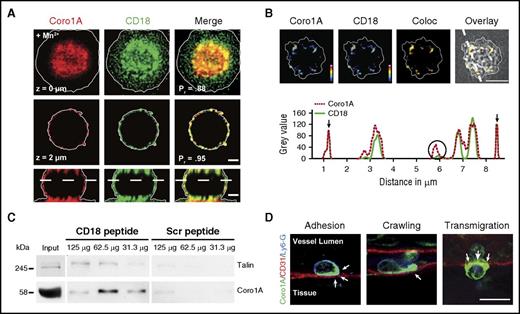
To test the hypothesis that Coro1A may represent a novel and previously unknown key molecule functionally linking integrins to the cytoskeleton, we set out to decipher the Coro1A interactome by coimmunoprecipitation and subsequent mass spectrometry by using human neutrophil-like differentiated HL-60 cells stably expressing Coro1A-EGFP (dHL-60-Coro1A-EGFP cells) (supplemental Figure 1A-B). The Coro1A interactome consisted of 95 proteins (supplemental Figure 2; supplemental Tables 1 and 2). One of the proteins, which only coprecipitated with Coro1A in adherent cells but not in suspended cells, was CD18 (ITGB2), the β subunit of the β2 integrins, suggesting a functional impact of Coro1A on PMN trafficking, which critically relies on β2 integrins. Indeed, Coro1A and CD18 colocalized at the sites of cell–substratum interactions as measured by CSRM and STED nanoscopy (Figure 1A-B; supplemental Video 1). At contact sites, Coro1A and CD18 showed unequivocal colocalization at the nanoscale. We verified the Coro1A–CD18 interaction in pull-down experiments using the synthesized cytoplasmic tail of CD18 incubated with cell lysates of freshly isolated human PMN (Figure 1C). Similar to Talin, a known CD18 interactor,28,29 Coro1A was pulled down by the CD18 cytoplasmic tail but not by the scrambled control peptide, indicating a specific interaction between Coro1A and the CD18 cytoplasmic tail in human neutrophils. During trauma-induced PMN trafficking in the mouse cremaster model, Coro1A was specifically enriched at critical regions, that is, at PMN–endothelial cell contact areas and in the lamellipodium during PMN adhesion, intraluminal crawling, and transendothelial migration (Figure 1D; supplemental Video 2). Similar results were obtained upon induction of inflammation by intrascrotal injection of rmTNF-α (supplemental Figure 1D), pointing toward a putative relevance of Coro1A for PMN trafficking during innate immunity.
Role of Coro1A in integrin biology. CSRM (A) and STED nanoscopy (B) of murine PMN upon Mn2+-induced adhesion to fibrinogen. (A) Coro1A (red), CD18 (green), and merge (yellow) in 2 different z-stack positions and in 1 orthogonal layer. Scale bar, 1 µm. Colocalization was calculated using the Pearson’s correlation coefficient (Pr). (B) Coro1A and CD18 in pseudocolors (colocalization in yellow) at surface proximal layer demonstrated by reflection pattern shown in transmission picture and intensity profile along segmented line. Partial (open circle) and complete (arrows) colocalization. Color scale, heat map. Scale bar, 5 μm. (C) Pull-down assay using synthesized peptides of the cytoplasmic tail of CD18 or scrambled control peptides incubated with different protein amounts of lysates of primary human PMNs (n = 3). (D) Subcellular localization of Coro1A (arrows) during trauma-induced PMN trafficking, that is, adhesion, intraluminal crawling, and transmigration in cremaster muscle whole mounts. Coro1A (green), CD31 (red), Ly6-G (blue). Scale bar, 10 µm. Coloc, colocalization; Scr, scrambled control peptides.
Role of Coro1A in integrin biology. CSRM (A) and STED nanoscopy (B) of murine PMN upon Mn2+-induced adhesion to fibrinogen. (A) Coro1A (red), CD18 (green), and merge (yellow) in 2 different z-stack positions and in 1 orthogonal layer. Scale bar, 1 µm. Colocalization was calculated using the Pearson’s correlation coefficient (Pr). (B) Coro1A and CD18 in pseudocolors (colocalization in yellow) at surface proximal layer demonstrated by reflection pattern shown in transmission picture and intensity profile along segmented line. Partial (open circle) and complete (arrows) colocalization. Color scale, heat map. Scale bar, 5 μm. (C) Pull-down assay using synthesized peptides of the cytoplasmic tail of CD18 or scrambled control peptides incubated with different protein amounts of lysates of primary human PMNs (n = 3). (D) Subcellular localization of Coro1A (arrows) during trauma-induced PMN trafficking, that is, adhesion, intraluminal crawling, and transmigration in cremaster muscle whole mounts. Coro1A (green), CD31 (red), Ly6-G (blue). Scale bar, 10 µm. Coloc, colocalization; Scr, scrambled control peptides.
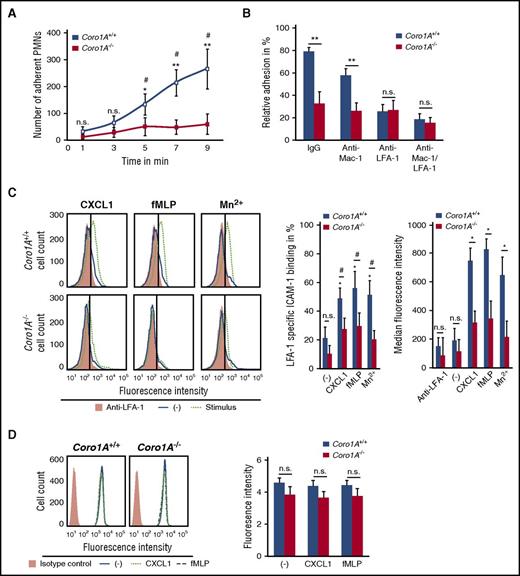
Coro1A controls PMN adhesion under flow conditions by regulating LFA-1 affinity
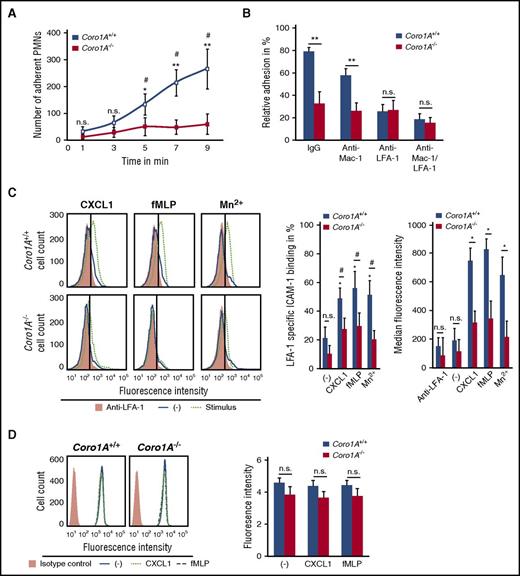
The functional impact of Coro1A for PMN trafficking was explored in a reductionist approach under defined flow conditions using microflow chambers coated with rmP-selectin, rmICAM-1, and rmCXCL1. Here, murine Coro1A+/+ PMNs adhered instantaneously upon rolling, whereas this response was almost completely absent in Coro1A−/− PMNs, as measured by time-lapse video microscopy (Figure 2A). Supplemental Video 3 illustrates this effect 9 minutes after the onset of the experiment: at this time point, adherent Coro1A+/+ PMNs already started to crawl, but Coro1A−/− PMNs were still rolling and almost unable to adhere. Interestingly, under static conditions, rmCXCL1-induced PMN adhesion on rmICAM-1- or fibrinogen-coated surfaces was intact in the genetic absence of Coro1A (supplemental Figure 3A). Similar results were obtained upon stimulation with fMLP or Mn2+, stabilizing β2 integrins in the high-affinity conformation.30 These results indicate that Coro1A was specifically required for PMN adhesion under flow but not under static conditions. Interestingly, the data were significant only at later time points but not within the first 2 minutes, suggesting an involvement of Coro1A in adhesion strengthening. As was expected, a function blocking anti-LFA-1 antibody31 (Figure 2B), but not a function blocking anti-Mac-1 antibody, significantly impaired induction of adhesion of Coro1A+/+ PMNs under flow conditions. When LFA-1 was blocked, the genetic absence of Coro1A had no additional effect in comparison with Coro1A+/+ PMNs, suggesting that Coro1A controls adhesion via LFA-1. Indeed, LFA-1 specific rmICAM-1 binding upon stimulation with rmCXCL1 or fMLP was severely reduced in Coro1A−/− PMNs in comparison with Coro1A+/+ PMNs, indicating a role of Coro1A in LFA-1 affinity regulation (Figure 2C). Even upon Mn2+ treatment, Coro1A−/− PMNs were unable to upregulate rmICAM-1 binding, suggesting an involvement of Coro1A in regulating LFA-1 affinity independent of inside-out signaling. Depolymerization of the F-actin cytoskeleton using cytochalasin B completely abrogated the ability of PMNs to upregulate LFA-1 affinity, confirming the requirement of cytoskeletal rearrangements for the induction of the high-affinity conformation of LFA-18 (supplemental Figure 3B), whereas LFA-1 cell surface expression of Coro1A−/− PMNs was not altered in comparison with Coro1A+/+ PMNs (Figure 2D).
Coro1A controls PMN adhesion under flow conditions by regulating LFA-1 affinity. (A) Induction of adhesion of Coro1A+/+ and Coro1A−/− PMNs under flow conditions (1 dyne/cm2) in microflow chambers coated with immobilized rmP-selectin, rmICAM-1, and rmCXCL1 at indicated times (n = 5). *P < .05; **P < .001 versus Coro1A−/− PMNs; #P < .05 versus initially adherent Coro1A+/+ PMNs at 1 minute. Mean ± standard deviation. (B) Induction of PMN adhesion in the presence of LFA-1 (clone M17/4, CD11a) or Mac-1 (clone M1/70, CD11b) function blocking or both, or control antibodies (IgG) under flow conditions (1 dyne/cm2). Relative adhesion of Coro1A+/+ and Coro1A−/− PMNs in percentage of all interacting PMNs (100%) after stimulation with rmCXCL1 for 9 minutes (n = 4). **P < .001. Mean ± standard deviation. (C) LFA-1 specific rmICAM-1 binding of Coro1A+/+ and Coro1A−/− PMNs stimulated for 3 minutes with 100 ng/mL of rmCXCL1, 10 μM of fMLP, 5 mM of Mn2+, or left unstimulated (minus sign). Mac-1 binding to rmICAM-1 was prevented by using a function blocking anti–Mac-1 antibody (clone M1/70). Fluorescence histograms (left) and quantitative analysis indicating percentage of cells positive for LFA-1 specific rmICAM-1-Fc binding in percentage of all PMNs (100%; middle), and median fluorescence intensity (right). Anti-LFA-1 antibody (clone M17/4, CD11a) was used to define negative control (n = 9, minus sign, unstimulated; n = 6, fMLP, rmCXCL1; n = 3, Mn2+). #P < .05; *P < .05 versus unstimulated control. Mean ± standard deviation. (D) CD11a cell surface expression of Coro1A+/+ and Coro1A−/− PMNs stimulated for 20 minutes with 100 ng/mL of rmCXCL1, 10 μM of fMLP, or left unstimulated (minus sign), isotype control (orange). Fluorescence histograms (left) and quantitative analysis (right) (n = 4). Mean ± standard deviation. n.s., not significant.
Coro1A controls PMN adhesion under flow conditions by regulating LFA-1 affinity. (A) Induction of adhesion of Coro1A+/+ and Coro1A−/− PMNs under flow conditions (1 dyne/cm2) in microflow chambers coated with immobilized rmP-selectin, rmICAM-1, and rmCXCL1 at indicated times (n = 5). *P < .05; **P < .001 versus Coro1A−/− PMNs; #P < .05 versus initially adherent Coro1A+/+ PMNs at 1 minute. Mean ± standard deviation. (B) Induction of PMN adhesion in the presence of LFA-1 (clone M17/4, CD11a) or Mac-1 (clone M1/70, CD11b) function blocking or both, or control antibodies (IgG) under flow conditions (1 dyne/cm2). Relative adhesion of Coro1A+/+ and Coro1A−/− PMNs in percentage of all interacting PMNs (100%) after stimulation with rmCXCL1 for 9 minutes (n = 4). **P < .001. Mean ± standard deviation. (C) LFA-1 specific rmICAM-1 binding of Coro1A+/+ and Coro1A−/− PMNs stimulated for 3 minutes with 100 ng/mL of rmCXCL1, 10 μM of fMLP, 5 mM of Mn2+, or left unstimulated (minus sign). Mac-1 binding to rmICAM-1 was prevented by using a function blocking anti–Mac-1 antibody (clone M1/70). Fluorescence histograms (left) and quantitative analysis indicating percentage of cells positive for LFA-1 specific rmICAM-1-Fc binding in percentage of all PMNs (100%; middle), and median fluorescence intensity (right). Anti-LFA-1 antibody (clone M17/4, CD11a) was used to define negative control (n = 9, minus sign, unstimulated; n = 6, fMLP, rmCXCL1; n = 3, Mn2+). #P < .05; *P < .05 versus unstimulated control. Mean ± standard deviation. (D) CD11a cell surface expression of Coro1A+/+ and Coro1A−/− PMNs stimulated for 20 minutes with 100 ng/mL of rmCXCL1, 10 μM of fMLP, or left unstimulated (minus sign), isotype control (orange). Fluorescence histograms (left) and quantitative analysis (right) (n = 4). Mean ± standard deviation. n.s., not significant.
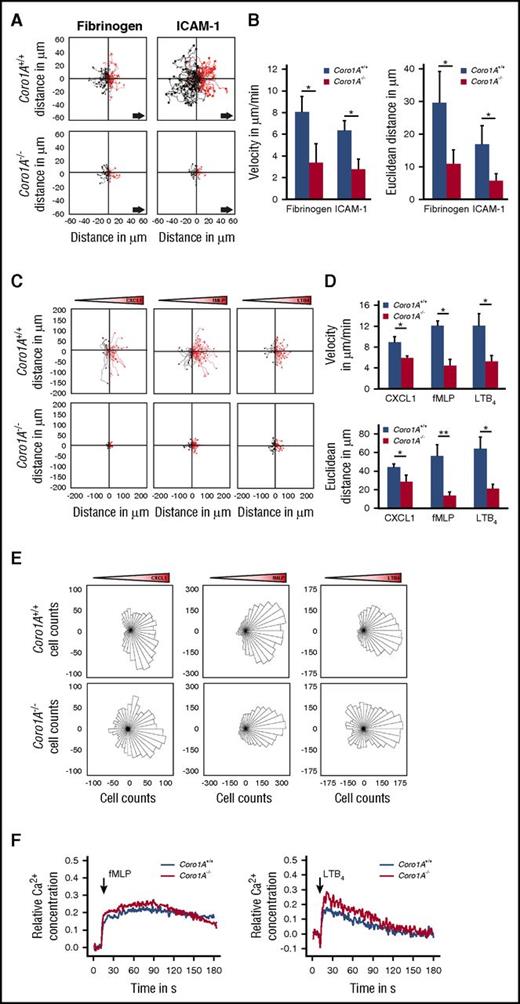
Coro1A impacts Mac-1 affinity regulation and PMN adhesion strengthening, spreading, and migration
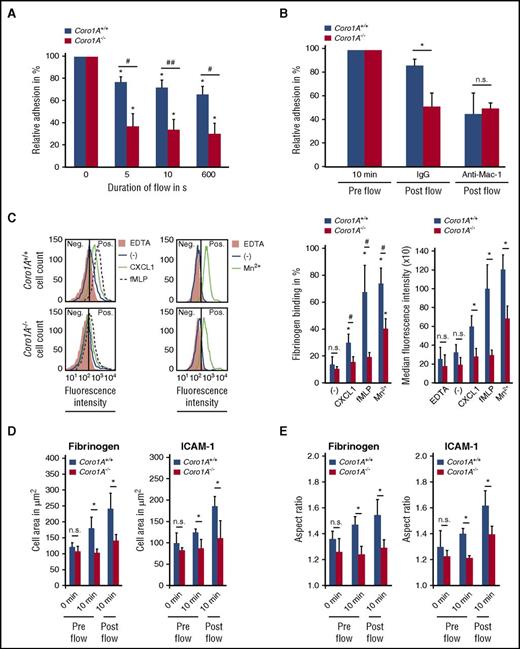
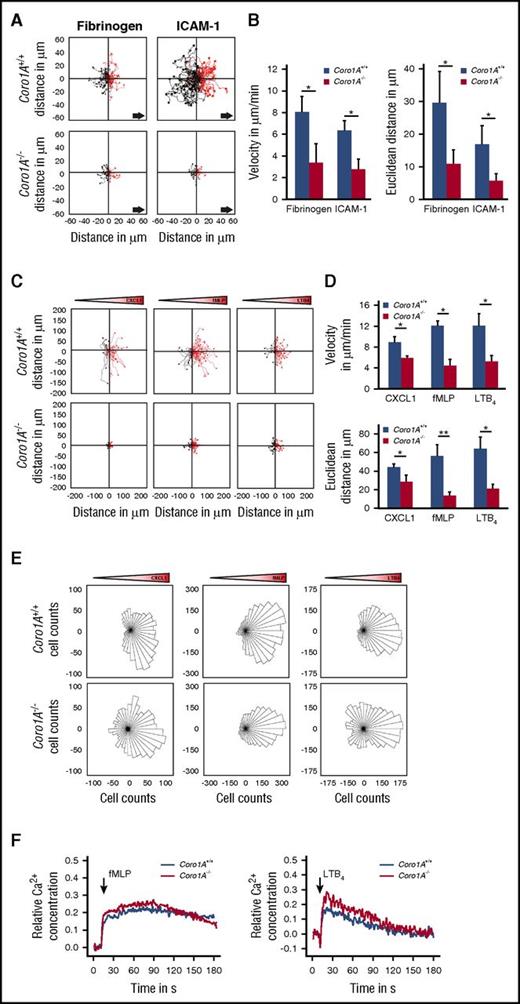
Additional experiments revealed the importance of Coro1A in PMN adhesion strengthening and Mac-1 affinity regulation (Figure 3A-C), as well as PMN spreading and polarization on immobilized fibrinogen and rmICAM-1 (Figure 3D-E; supplemental Video 3), whereas Mac-1 expression was similar in Coro1A+/+ and Coro1A−/− PMNs (supplemental Figure 4F). As was expected, a function blocking anti-Mac-1 antibody (Figure 3B), but not an isotype control antibody, significantly impaired adhesion strengthening of Coro1A+/+ PMNs under flow conditions. When Mac-1 was blocked, the genetic absence of Coro1A had no additional effect in comparison with Coro1A+/+ PMNs, suggesting that Coro1A controls adhesion strengthening via Mac-1 (Figure 3B). Moreover, the quantitative analysis of mechanotactic migration in microflow chambers coated with fibrinogen or rmICAM-1 unraveled the functional importance of Coro1A for this process (Figure 4A-B). Here, migration velocity and the Euclidean distance were significantly reduced in the genetic absence of Coro1A. Accordingly, chemotactic migration in Zigmond chambers, that is, migration velocities and the Euclidean distance in response to gradients of rmCXCL1, fMLP, or LTB4, was dramatically reduced in Coro1A−/− PMNs in comparison with Coro1A+/+ PMNs (Figure 4C-D). Detailed analysis of single-cell migration tracks using rose diagrams showed that Coro1−/− PMNs still oriented toward the source of the chemoattractants, similar to Coro1A+/+ PMNs (Figure 4E). Thus, the absence of Coro1A compromised the migratory capacity of PMNs but not the sensing of and orientation toward the chemotactic gradient. This observation was in accordance to the finding that fMLP- or LTB4-induced Ca2+ signaling was intact in Coro1A−/− PMNs, suggesting that G protein-coupled receptor (GPCR)-proximal signaling was not affected by the absence of Coro1A (Figure 4F).
Mac-1 affinity regulation via Coro1A impacts PMN adhesion strengthening and spreading. (A) Quantitative analysis of adherent Coro1A+/+ and Coro1A−/− PMNs under flow conditions (1 dyne/cm2) on immobilized fibrinogen in percentage of adherent cells prior to onset of shear stress (100%) at indicated time points (n = 4). #P < .05; ##P < .001; *P < .05 versus initially adherent cells. Mean ± standard deviation. (B) Adhesion strengthening of Coro1A+/+ and Coro1A−/− PMNs on immobilized fibrinogen in the presence of a function blocking anti-Mac-1 antibody (clone M1/70, CD11b) or an isotype control antibody (IgG) under flow conditions (1 dyne/cm2). Relative adhesion of Coro1A+/+ and Coro1A−/− PMNs in percentage of all interacting PMNs prior to onset of shear stress (pre flow, 100%) (n = 3). *P < .05. Mean ± standard deviation. (C) Flow cytometric analysis of soluble fibrinogen binding to Coro1A+/+ and Coro1A−/− PMNs stimulated for 20 minutes with 100 ng/mL of rmCXCL1, 10 μM of fMLP, 5 mM of Mn2+, or left unstimulated (minus sign). Percentage of PMN positive for fibrinogen binding was calculated by defining a threshold of fluorescence intensity at which 95% of PMN in the EDTA control were considered negative. Fluorescence histograms (left) and quantitative analysis showing percentage (middle) and median fluorescence intensity (right) of cells with positive fibrinogen binding (n = 6, rmCXCL1, fMLP; n = 3, Mn2+). #P < .05; *P < .05 versus unstimulated control. Mean ± standard deviation. (D-E) The fMLP-induced spreading and polarization of Coro1A+/+ and Coro1A−/− PMNs upon exposure to immobilized fibrinogen or rmICAM-1 under static conditions (pre flow) or after application of flow (post flow, 1 dyne/cm2) at indicated time points as calculated by measuring the cell area (D) and the aspect ratio (E) (n = 4, fibrinogen, ≥68 Coro1A+/+ and ≥55 Coro1A−/− PMNs; n = 3, rmICAM-1, ≥53 Coro1A+/+ and ≥50 Coro1A−/− PMNs). Mean ± standard deviation. *P < .05.
Mac-1 affinity regulation via Coro1A impacts PMN adhesion strengthening and spreading. (A) Quantitative analysis of adherent Coro1A+/+ and Coro1A−/− PMNs under flow conditions (1 dyne/cm2) on immobilized fibrinogen in percentage of adherent cells prior to onset of shear stress (100%) at indicated time points (n = 4). #P < .05; ##P < .001; *P < .05 versus initially adherent cells. Mean ± standard deviation. (B) Adhesion strengthening of Coro1A+/+ and Coro1A−/− PMNs on immobilized fibrinogen in the presence of a function blocking anti-Mac-1 antibody (clone M1/70, CD11b) or an isotype control antibody (IgG) under flow conditions (1 dyne/cm2). Relative adhesion of Coro1A+/+ and Coro1A−/− PMNs in percentage of all interacting PMNs prior to onset of shear stress (pre flow, 100%) (n = 3). *P < .05. Mean ± standard deviation. (C) Flow cytometric analysis of soluble fibrinogen binding to Coro1A+/+ and Coro1A−/− PMNs stimulated for 20 minutes with 100 ng/mL of rmCXCL1, 10 μM of fMLP, 5 mM of Mn2+, or left unstimulated (minus sign). Percentage of PMN positive for fibrinogen binding was calculated by defining a threshold of fluorescence intensity at which 95% of PMN in the EDTA control were considered negative. Fluorescence histograms (left) and quantitative analysis showing percentage (middle) and median fluorescence intensity (right) of cells with positive fibrinogen binding (n = 6, rmCXCL1, fMLP; n = 3, Mn2+). #P < .05; *P < .05 versus unstimulated control. Mean ± standard deviation. (D-E) The fMLP-induced spreading and polarization of Coro1A+/+ and Coro1A−/− PMNs upon exposure to immobilized fibrinogen or rmICAM-1 under static conditions (pre flow) or after application of flow (post flow, 1 dyne/cm2) at indicated time points as calculated by measuring the cell area (D) and the aspect ratio (E) (n = 4, fibrinogen, ≥68 Coro1A+/+ and ≥55 Coro1A−/− PMNs; n = 3, rmICAM-1, ≥53 Coro1A+/+ and ≥50 Coro1A−/− PMNs). Mean ± standard deviation. *P < .05.
Coro1A is required for efficient migration of PMNs but is dispensable for GPCR-proximal Ca2+signaling of PMNs. (A-B) Mechanotactic migration of Coro1A+/+ and Coro1A−/− PMNs under flow (1 dyne/cm2) using microflow chambers coated with immobilized fibrinogen or rmICAM-1 in the presence of 10 µM of fMLP. (A) Single-cell migration tracks after 10 minutes of flow. Arrows indicate direction of flow. (B) Mean migration velocity and mean Euclidean distance (n = 4, fibrinogen; n = 3, rmICAM-1). Mean ± standard deviation. (C-E) Chemotactic migration of Coro1A+/+ and Coro1A−/− PMNs toward gradients of 100 ng/mL of rmCXCL1, 10 µM of fMLP, or 100 nM of LTB4 using Zigmond chambers. Gradient cones indicate orientation of gradients. (C) Single-cell migration tracks. (D) Mean migration velocity and mean Euclidean distance are graphed (n = 3). Mean ± standard deviation. (E) Rose diagrams. The area of each sector is proportional to the frequency of the migration vectors of tracked Coro1A+/+ and Coro1A−/− PMNs pointed in the respective direction in response to gradients of rmCXCL1, fMLP, or LTB4. Rose plots shown are representative of 3 independent experiments. (F) Ca2+ signaling in Coro1A+/+ and Coro1A−/− PMNs upon stimulation with 10 µM of fMLP (left) or 100 nM of LTB4 (right). Cytosolic Ca2+ concentration was measured in Fura-2 AM-labeled PMNs. Relative concentration was determined in percentage of maximum Ca2+ response upon treatment with 12 µM of ionomycin. Data are representative of 4 independent experiments. *P < .05.
Coro1A is required for efficient migration of PMNs but is dispensable for GPCR-proximal Ca2+signaling of PMNs. (A-B) Mechanotactic migration of Coro1A+/+ and Coro1A−/− PMNs under flow (1 dyne/cm2) using microflow chambers coated with immobilized fibrinogen or rmICAM-1 in the presence of 10 µM of fMLP. (A) Single-cell migration tracks after 10 minutes of flow. Arrows indicate direction of flow. (B) Mean migration velocity and mean Euclidean distance (n = 4, fibrinogen; n = 3, rmICAM-1). Mean ± standard deviation. (C-E) Chemotactic migration of Coro1A+/+ and Coro1A−/− PMNs toward gradients of 100 ng/mL of rmCXCL1, 10 µM of fMLP, or 100 nM of LTB4 using Zigmond chambers. Gradient cones indicate orientation of gradients. (C) Single-cell migration tracks. (D) Mean migration velocity and mean Euclidean distance are graphed (n = 3). Mean ± standard deviation. (E) Rose diagrams. The area of each sector is proportional to the frequency of the migration vectors of tracked Coro1A+/+ and Coro1A−/− PMNs pointed in the respective direction in response to gradients of rmCXCL1, fMLP, or LTB4. Rose plots shown are representative of 3 independent experiments. (F) Ca2+ signaling in Coro1A+/+ and Coro1A−/− PMNs upon stimulation with 10 µM of fMLP (left) or 100 nM of LTB4 (right). Cytosolic Ca2+ concentration was measured in Fura-2 AM-labeled PMNs. Relative concentration was determined in percentage of maximum Ca2+ response upon treatment with 12 µM of ionomycin. Data are representative of 4 independent experiments. *P < .05.
Coro1A is critical for PMN trafficking in innate immunity
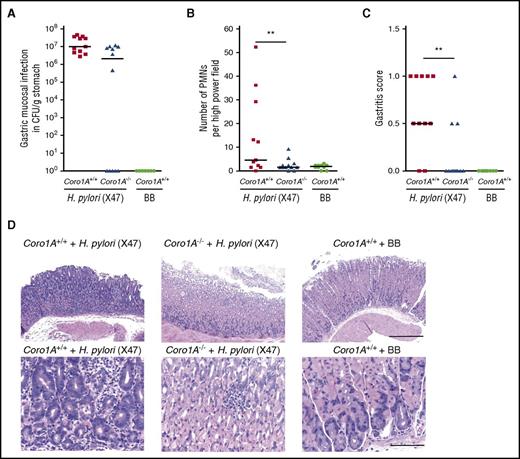
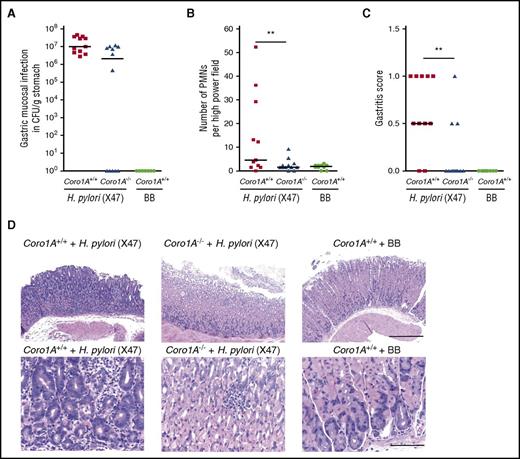
In vivo imaging of mouse cremaster muscle venules revealed profound defects in the induction of leukocyte adhesion, intraluminal crawling, and extravasation upon fMLP superfusion in Coro1A−/− mice, as measured by conventional and 2-photon intravital microscopy (IVM) (Figure 5A-F; supplemental Video 4). Total white blood cell counts in Coro1A−/− mice were decreased because of diminished lymphocyte counts, as was previously reported,5 whereas PMN maturation, life span, and blood cell counts were similar in Coro1A−/− and Coro1A+/+ mice (supplemental Figure 4). Leukocyte rolling and rolling velocities were similar in both mouse strains (Figure 5A-B). This was also true for rheological parameters (supplemental Table 3). Similar results were obtained upon intrascrotal injection of rmTNF-α (supplemental Figure 5; supplemental Table 3). To study the potential impact of Coro1A for PMN trafficking in a microbial infection model, we chose the stomach-colonizing human bacterial pathogen H pylori. Coro1A+/+ and Coro1A−/− mice were infected with the mouse-adapted H pylori strain (X47) or treated with BB medium for control. Whereas all H pylori X47-challenged Coro1A+/+ mice were successfully infected, this was only true for 7 out of 12 Coro1A−/− mice (Figure 6A). However, PMN accumulation in the gastric submucosa was significantly reduced in Coro1A−/− mice in comparison with Coro1A+/+ animals (Figure 6B). The gastric mucosa of Coro1A+/+ mice treated with BB medium was completely free of signs of infection (Figure 6A-D). Gastritis scores26 were significantly lower in Coro1A−/− mice in comparison with Coro1A+/+ mice (Figure 6C), demonstrating the functional importance of Coro1A for PMN accumulation at sites of lesion and thereby significantly affecting the severity of gastritis upon infection with the bacterial pathogen H pylori.
Coro1A is critical for PMN trafficking in innate immunity. (A-F) IVM of fMLP-superfused postcapillary cremaster muscle venules of Coro1A+/+ and Coro1A−/− mice. (A) Rolling leukocytes before (0 minutes) and 15 minutes after fMLP superfusion (n = 18 vessels per 5 mice, Coro1A+/+; n = 16 vessels per 5 mice; Coro1A−/−). Mean ± standard error of the mean. (B) Cumulative frequency distribution of leukocyte rolling velocities (n = 169 cells per 18 vessels per 5 mice, Coro1A+/+; n = 154 cells per 16 vessels per 5 mice, Coro1A−/−). (C) Adherent leukocytes before (0 minute) and during fMLP superfusion for indicated time periods (n = 9 vessels per 5 mice, Coro1A+/+; n = 9 vessels per 5 mice, Coro1A−/−). Mean ± standard error of the mean. (D) Mean velocity and mean Euclidean distance of PMNs during intraluminal crawling (n = 538 cells per 11 vessels per 3 mice, Coro1A+/+; n = 232 cells per 21 vessels per 6 mice, Coro1A−/−). Mean ± standard error of the mean. (E) Microscopic images after 70 minutes of fMLP superfusion. Interstitial PMN migration routes were tracked. Scale bar, 30 µm. (F) Interstitial PMN accumulation after fMLP superfusion for indicated time periods (n ≥ 319 cells per 29 vessels per 5 mice, Coro1A+/+; n ≥ 104 cells per 35 vessels per 7 mice, Coro1A−/−). Mean ± standard error of the mean.*P < .05 versus Coro1A+/+ mice.
Coro1A is critical for PMN trafficking in innate immunity. (A-F) IVM of fMLP-superfused postcapillary cremaster muscle venules of Coro1A+/+ and Coro1A−/− mice. (A) Rolling leukocytes before (0 minutes) and 15 minutes after fMLP superfusion (n = 18 vessels per 5 mice, Coro1A+/+; n = 16 vessels per 5 mice; Coro1A−/−). Mean ± standard error of the mean. (B) Cumulative frequency distribution of leukocyte rolling velocities (n = 169 cells per 18 vessels per 5 mice, Coro1A+/+; n = 154 cells per 16 vessels per 5 mice, Coro1A−/−). (C) Adherent leukocytes before (0 minute) and during fMLP superfusion for indicated time periods (n = 9 vessels per 5 mice, Coro1A+/+; n = 9 vessels per 5 mice, Coro1A−/−). Mean ± standard error of the mean. (D) Mean velocity and mean Euclidean distance of PMNs during intraluminal crawling (n = 538 cells per 11 vessels per 3 mice, Coro1A+/+; n = 232 cells per 21 vessels per 6 mice, Coro1A−/−). Mean ± standard error of the mean. (E) Microscopic images after 70 minutes of fMLP superfusion. Interstitial PMN migration routes were tracked. Scale bar, 30 µm. (F) Interstitial PMN accumulation after fMLP superfusion for indicated time periods (n ≥ 319 cells per 29 vessels per 5 mice, Coro1A+/+; n ≥ 104 cells per 35 vessels per 7 mice, Coro1A−/−). Mean ± standard error of the mean.*P < .05 versus Coro1A+/+ mice.
Genetic absence of Coro1A significantly reduces the severity of H pylori–induced gastritis. (A-D) Coro1A+/+ and Coro1A−/− mice were infected with H pylori X47 or inoculated with BB medium. H pylori–inoculated Coro1A+/+ mice (solid squares, n = 11) and Coro1A−/− mice (solid triangles, n = 12), BB medium–inoculated Coro1+/+ mice (solid circles, n = 10). (A) Colonization of H pylori (log CFUs per gram of gastric tissue). (B) Number of PMNs in the high power field. (C) Gastritis scores.26 (D) Representative histological sections of gastric mucosa stained with hematoxylin and eosin. Scale bars, 400 µm (top) and 100 µm (bottom). **P < .001.
Genetic absence of Coro1A significantly reduces the severity of H pylori–induced gastritis. (A-D) Coro1A+/+ and Coro1A−/− mice were infected with H pylori X47 or inoculated with BB medium. H pylori–inoculated Coro1A+/+ mice (solid squares, n = 11) and Coro1A−/− mice (solid triangles, n = 12), BB medium–inoculated Coro1+/+ mice (solid circles, n = 10). (A) Colonization of H pylori (log CFUs per gram of gastric tissue). (B) Number of PMNs in the high power field. (C) Gastritis scores.26 (D) Representative histological sections of gastric mucosa stained with hematoxylin and eosin. Scale bars, 400 µm (top) and 100 µm (bottom). **P < .001.
Coro1A as a novel player in integrin regulation and F-actin dynamics
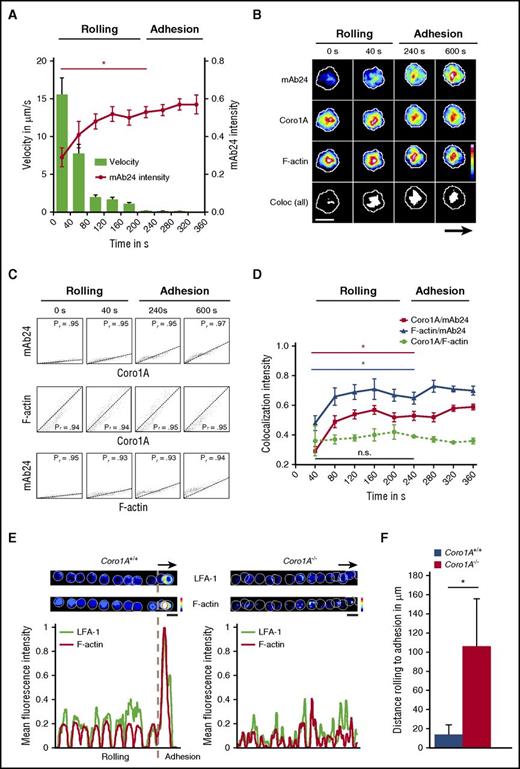
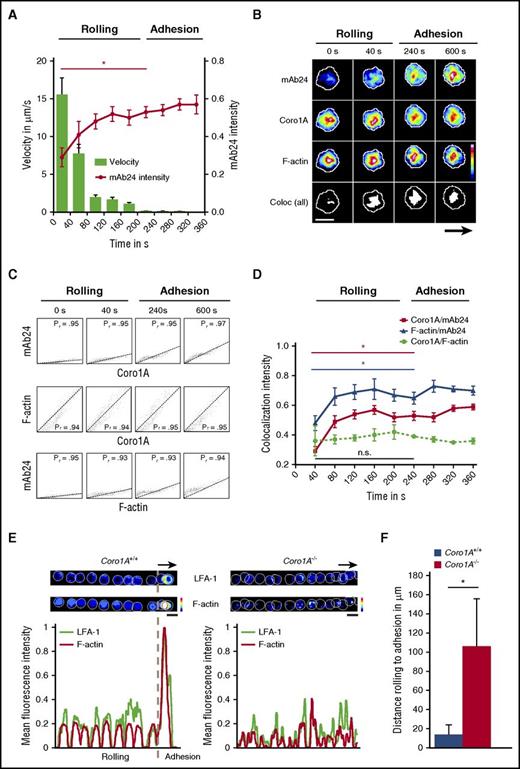
To explore the functional impact of Coro1A for the spatial and temporal dynamics of integrin affinity regulation during PMN trafficking, namely adhesion and migration, we combined live-cell spinning-disk confocal microscopy with microflow chamber assays using dHL-60-Coro1A-EGFP cells. During the process of leukocyte rolling, the rolling velocities declined gradually, finally resulting in the induction of adhesion after ∼200 seconds (Figure 7A). The process of leukocyte rolling was characterized by a cumulative increase of mAb24 antibody intensity over time, reporting a gradual rise of the number of β2 integrins in the high-affinity conformation on the cell surface,32,33 representing the key event critical for the transition of leukocyte rolling to firm adhesion. At this time point, mAb24 antibody intensity was significantly increased in comparison with the values observed in rolling leukocytes. Strikingly, the spatiotemporal dynamics of this process revealed unequivocally a strong colocalization of integrins in the high-affinity confirmation with Coro1A, as measured by live-cell imaging and calculated by the Pearson’s correlation coefficient and the Manders’ colocalization coefficient, verified by the Costes method34,35 (Figure 7B-C; supplemental Figure 6; supplemental Video 5). The colocalization between Coro1A and F-actin was constitutively present during the whole observation period, including leukocyte rolling and adhesion, and the intensity of colocalization did not change over time (Figure 7B-D; supplemental Video 6). When integrins with high-affinity conformation appeared during the recruitment process, these molecules were always colocalized with Coro1A and the F-actin cytoskeleton, whereas the intensity of mAb24 increased over time, reflecting the accumulating number of integrin molecules in the high-affinity state (Figure 7C-D; supplemental Figure 6). Moreover, the transition from rolling to adhesion was accompanied by LFA-1 clustering and F-actin accumulation in Coro1A+/+ PMNs, whereas the majority of Coro1A−/− PMNs failed to adhere, cluster LFA-1, and accumulate F-actin (Figure 7E). This dysregulation led to prolonged rolling distances until the onset of adhesion (Figure 7F). Similar results were obtained during the process of migration, during which integrins in the high-affinity conformation almost completely colocalized with Coro1A and the F-actin cytoskeleton during integrin-mediated adhesive interactions (supplemental Figures 6 and 7; supplemental Video 7). However, not all Coro1A molecules were associated with LFA-1 during the process of migration, reflecting putative additional roles of Coro1A, as suggested by its redistribution to, for example, the lamellipodium of the polarized cell (supplemental Figures 1C and 7). In conclusion, the actin-binding protein Coro1A constitutively interacted with the cortical F-actin cytoskeleton during the process of leukocyte rolling. The induction of the high-affinity conformation of LFA-1, which allows the transition of leukocyte rolling to firm adhesion, involved the association of Coro1A with CD18, the β subunit of the β2 integrins. Accordingly, the absence of Coro1A compromised affinity regulation and clustering of LFA-1 and impaired firm PMN adhesion, pointing toward a novel and unexpected key function of Coro1A in integrin biology by fine-tuning the actin cytoskeleton, which is critical for PMN trafficking in innate immunity.
Coro1A controls affinity regulation and clustering of LFA-1 critical for PMN adhesion under flow. In vitro flow chamber assays were performed with dHL-60-Coro1A-EGFP cells by using spinning-disk confocal microscopy. (A) Kinetics of induction of β2 integrin high-affinity conformation were analyzed by binding of mAb24 antibody. Single-cell velocity and mAb24 intensity values were measured at indicated time points. Data of 3 representative cells from 3 independent experiments are shown. Mean ± standard deviation. (B) Representative pseudocolored snapshots demonstrating the subcellular localization of high-affinity β2 integrins (mAb24), Coro1A, F-actin, and colocalization of all 3 molecules (white) during rolling and adhesion in single-cell analysis. Arrow indicates direction of flow. (C) Scatter plots of high-affinity LFA-1, Coro1A, and F-actin intensity profiles in single-cell analysis. Colocalization was monitored by calculating the Pearson’s correlation coefficient (Pr) at indicated time points. (D) Intensity profiles of colocalized Coro1A/mAb24, mAb24/F-actin, and Coro1A/F-actin complexes during rolling and adhesion. Data of 3 representative cells from 3 independent experiments are shown. Mean ± standard deviation. (E) Representative pseudocolored overlay of single time points and intensity profiles demonstrating LFA-1 clustering using the Alexa Fluor 594-conjugated anti-CD11a antibody (clone 2D7) and F-actin accumulation in Coro1A+/+ and Coro1A−/− PMNs during rolling and adhesion. Arrow indicates direction of flow. A representative PMN from 3 independent experiments with a total of 33 Coro1A+/+ and 34 Coro1A−/− PMNs is shown. (F) Quantitative analysis of rolling distance until the onset of adhesion in Coro1A+/+ and Coro1A−/− PMNs (n = 3 with a total of 137 Coro1A+/+ and 24 Coro1A−/− PMNs). Mean ± standard deviation. Scale bars, 10 µm. Color scales, heat maps. *P < .05.
Coro1A controls affinity regulation and clustering of LFA-1 critical for PMN adhesion under flow. In vitro flow chamber assays were performed with dHL-60-Coro1A-EGFP cells by using spinning-disk confocal microscopy. (A) Kinetics of induction of β2 integrin high-affinity conformation were analyzed by binding of mAb24 antibody. Single-cell velocity and mAb24 intensity values were measured at indicated time points. Data of 3 representative cells from 3 independent experiments are shown. Mean ± standard deviation. (B) Representative pseudocolored snapshots demonstrating the subcellular localization of high-affinity β2 integrins (mAb24), Coro1A, F-actin, and colocalization of all 3 molecules (white) during rolling and adhesion in single-cell analysis. Arrow indicates direction of flow. (C) Scatter plots of high-affinity LFA-1, Coro1A, and F-actin intensity profiles in single-cell analysis. Colocalization was monitored by calculating the Pearson’s correlation coefficient (Pr) at indicated time points. (D) Intensity profiles of colocalized Coro1A/mAb24, mAb24/F-actin, and Coro1A/F-actin complexes during rolling and adhesion. Data of 3 representative cells from 3 independent experiments are shown. Mean ± standard deviation. (E) Representative pseudocolored overlay of single time points and intensity profiles demonstrating LFA-1 clustering using the Alexa Fluor 594-conjugated anti-CD11a antibody (clone 2D7) and F-actin accumulation in Coro1A+/+ and Coro1A−/− PMNs during rolling and adhesion. Arrow indicates direction of flow. A representative PMN from 3 independent experiments with a total of 33 Coro1A+/+ and 34 Coro1A−/− PMNs is shown. (F) Quantitative analysis of rolling distance until the onset of adhesion in Coro1A+/+ and Coro1A−/− PMNs (n = 3 with a total of 137 Coro1A+/+ and 24 Coro1A−/− PMNs). Mean ± standard deviation. Scale bars, 10 µm. Color scales, heat maps. *P < .05.
Discussion
Unexpectedly, we identified Coro1A as a novel regulator of β2 integrins during PMN trafficking. CSRM and STED nanoscopy revealed an enrichment of Coro1A at adhesive sites during cell–substratum interactions where Coro1A colocalized with CD18. Moreover, pull-down experiments revealed an interaction of Coro1A with the cytoplasmic tail of CD18. Coro1A was enriched at contact sites between PMNs and the inflamed endothelium of murine cremaster muscle postcapillary venules during adhesion, crawling, and transmigration. Detailed functional analyses unraveled the impact of Coro1A for LFA-1- and Mac-1-dependent functions, including induction of adhesion, adhesion strengthening, spreading, and migration by mediating integrin affinity regulation, a key event in CXCL1 signaling that activates LFA-1 and thereby allows PMN adhesion under flow conditions.9,36 Interestingly, this effect was also observed upon treatment with Mn2+ emphasizing the importance of Coro1A for LFA-1 affinity regulation when bypassing integrin inside-out signaling. The disruption of the F-actin cytoskeleton upon cytochalasin B treatment abrogated LFA-1 affinity regulation, suggesting an interdependence of integrins, the cytoskeleton, and Coro1A during this process. In contrast, PMN adhesion under static conditions was unaffected in the genetic absence of Coro1A, demonstrating a specific role for this molecule when fine-tuning of the cytoskeleton is critically required. In a manner similar to that of LFA-1, Mac-1-mediated PMN functions including adhesion strengthening, spreading, and polarization as well as Mac-1 affinity regulation were severely compromised in Coro1A−/− PMNs when compared with Coro1A+/+ PMNs. Analysis of mechanotactic crawling and chemotactic migration demonstrated that the general migratory capacity of PMNs was severely impaired in the absence of Coro1A, which is in line with findings by Yan et al.17 This was in contrast to a study by Combulazier et al, who found no defects in chemotaxis in the absence of Coro1A in PMNs.37 Here, transwell assays were used with a pore size of 8 µm, which may have led to the negative results. In our experiments, the direction of migration under flow conditions, as well as the orientation of Coro1A−/− PMNs toward the source of the chemoattractant gradient, were unaffected when compared with Coro1A+/+ PMNs. These observations are in line with our findings that Ca2+ release in Coro1A−/− PMNs was normal upon fMLP or LTB4 stimulation, indicating that Coro1A was dispensable for GPCR-induced proximal signaling in PMNs. Notably, Ca2+ responses were severely impaired in the absence of Coro1A upon B-cell and T-cell receptor stimulation, resulting in impaired T-cell survival and T-cell homeostasis.5,38 Thus, the biological significance of Coro1A in regard to Ca2+ signaling has evolved differently in adaptive and innate immunity.39
During PMN rolling on rmP-selectin and rmICAM-1, Coro1A was associated with the F-actin cytoskeleton. The process of deceleration toward firm adhesion was characterized by increasing amounts of high-affinity LFA-1 on the cell surface, colocalizing with Coro1A and F-actin at local adhesion sites, termed focal zones.40 Similarly, Coro1A and F-actin were located at focal zones during mechanotactic crawling. Remarkably, almost all high-affinity LFA-1 was constantly colocalized with Coro1A and F-actin during all these steps, suggesting that the induction, persistence, or both of high-affinity LFA-1 was highly dependent on Coro1A, possibly by mediating the functional interaction of the integrins with the F-actin cytoskeleton (supplemental Figure 8).
Intravital microscopy revealed that the number of rolling leukocytes as well as rolling velocities were unaffected in Coro1A−/− mice when compared with Coro1A+/+ mice, suggesting that Coro1A was dispensable for induction of LFA-1 intermediate affinity during leukocyte rolling, which is independent of the F-actin cytoskeleton.7,8 In sharp contrast, leukocyte adhesion and extravasation were dramatically impaired in Coro1A−/− mice when compared with Coro1A+/+ mice. Furthermore, PMN intraluminal crawling and extravasation were severely compromised in the absence of Coro1A, as assessed by 2-photon intravital microscopy during fMLP-induced inflammation. Taken together, these results demonstrate the crucial role of Coro1A for β2 integrin-dependent PMN recruitment steps that require high-affinity integrins, integrin clustering, and cytoskeletal reorganization. Strikingly, in a laser-induced ear injury model of sterile inflammation, we observed no difference in the inflammatory response between Coro1A−/− and Coro1A+/+ mice (data not shown), suggesting tissue-specific or stimulus-dependent functional importance of Coro1A for PMN trafficking in the innate immune response. These observations are in line with previous findings by Siegmund et al, who used concanavalin A–induced liver injury as a model for sterile inflammation, suggesting that damage-associated molecular patterns that trigger PMN recruitment under sterile conditions do not depend on Coro1A.41,42 In addition, PMN maturation, life span, and blood cell counts were similar in Coro1A−/− and Coro1A+/+ mice, whereas T-cell homeostasis in mice and PMN homeostasis in the human system are altered in the absence of Coro1A.5,43
In the pathophysiological context of an H pylori infection,44 the genetic deficiency of Coro1A resulted in a dramatic reduction of PMN infiltration into the gastric mucosa and an almost complete protection from development of gastritis, when compared with Coro1A+/+ mice. Approximately, 50% of the human population is chronically infected with H pylori, causing mucosal damage ranging from mild gastritis, to stomach ulcers, to adenocarcinomas.23,45 The severity of the disease correlates with the magnitude of PMN recruitment elicited by H pylori–neutrophil-activating protein, one of the major virulence factors of H pylori that was shown to be chemotactic for PMN, to upregulate β2 integrin cell surface expression, and to activate β2 integrins into the high-affinity state.46-48 A gastritis score of 1, as usually found in H pylori X47 mouse stomach infection, is characterized by a mild infiltrate of inflammatory cells along the base of the glands, mostly PMNs, but also T cells. However, we cannot differentiate between a direct effect of reduced PMN infiltration of the gastric submucosa and an altered T-cell population and thus a change in the local cytokine profile affecting the gastritis score. Interestingly, only 58% of Coro1A−/− mice were successfully infected with the H pylori strain X47 in our study. This is in line with previous findings that H pylori recruits Coro1A to the phagosome upon bacterial ingestion, thereby inhibiting the fusion with lysosomes, which results in the protection from bacterial killing and enhanced survival of H pylori.49 Thus, the decreased susceptibility to infection observed in our studies may be due to the fact that the absence of Coro1A facilitated bacterial killing,19 pointing toward a dual role of Coro1A in H pylori infection. Our study demonstrates a fundamental role of Coro1A for PMN trafficking during innate immunity and thereby provides new molecular insights to this process. Interestingly, Coro1A function is controlled by phosphorylation, and dephosphorylation allows its activation and association with the F-actin cytoskeleton.50 Thus, the Coro1A pathway represents a druggable target, and inhibition of Coro1A function may represent an interesting novel therapeutic concept for the treatment of inflammatory diseases. Especially in H pylori infection, targeting Coro1A may not only decrease the susceptibility to bacterial infection but also prevent inflammation and disease progression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Reinhard Fässler, Max Planck Institute of Biochemistry, Martinsried, Germany, for his generous support and advice in CD18 pull-down experiments by sharing relevant tools, protocols, and expertise. The authors thank Tanja Vlaovic, Jennifer Truong, Severin Gylstorff, Marc Wirth, Susanne Bierschenk, and Eva Loell for their excellent technical assistance, as well as Steffen Dietzel (Core Facility Bioimaging, Biomedical Center, Ludwig-Maximilians-Universität München) for his help with CSRM and STED nanoscopy.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 914, project A02 [B.W.], A05 [R.T.B.], B01 [M. Sperandio], B02 [S.M.], B05 [R.H.], Z01 [A.G.K. and S.M.], and Z03 [B.W. and M. Sperandio]) and by the Förderprogramm für Forschung und Lehre (FöFoLe) of the Ludwig-Maximilians-Universität München.
Authorship
Contribution: R.P. performed in vitro experiments, including spinning-disk confocal microscopy, CSRM, and STED nanoscopy, analyzed the data, and wrote the manuscript; D. Begandt designed the experiments, analyzed the data, and wrote the manuscript; T.J.S. performed flow cytometry and intravital microscopy and analyzed the data; T.J.S. and M. Sperandio analyzed the in vivo data; M. Salvermoser, I.F., and A.I. performed coimmunoprecipitation experiments and analyzed mass spectrometry data; I.F. performed mass spectrometry; S.T. and R.T.B. performed pull-down experiments; E.M. and M. Salvermoser performed confocal imaging; U.H. and R.H. performed H pylori infection experiments and analyzed the data; R.C., A.G.K., and S.M. performed 2-photon intravital microscopy and analyzed the data; L.T.W. and D. Brechtefeld performed in vitro experiments and analyzed the data; and B.W. designed the overall study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara Walzog, Department of Cardiovascular Physiology and Pathophysiology, Walter Brendel Center of Experimental Medicine, Biomedical Center, Ludwig-Maximilians-Universität München, 82152 Planegg-Martinsried, Germany; e-mail: walzog@lrz.uni-muenchen.de.
References
Author notes
R.P. and D.B. are joint first authors.