In this issue of Blood, Konar and Granoff describe an elegant series of experiments that address the reason immunization against meningococcal disease can fail to protect people treated with the complement-blocking antibody, eculizumab.1
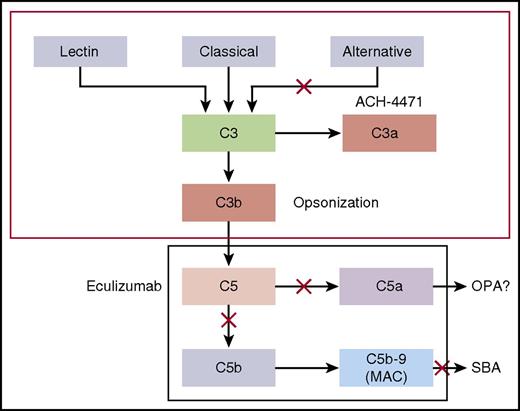
Model by which the classical and alternative complement pathways mediate SBA or OPA killing of meningococci. Blocking cleavage of C5 or C7 in the terminal complement pathway prevents formation of MAC, which is required for complement-mediated SBA. In the absence of MAC, anti-meningococcal antibodies may offer protection by eliciting antibody-Fc–mediated and/or C3b/iC3b complement receptor–mediated OPA. However, complement receptor–mediated phagocytic uptake as well as phagocytic cell chemotaxis is promoted by C5a, a split product of C5. Thus, blockage of release of C5a by eculizumab could abrogate this additional protective mechanism. See Figure 1 in the article by Konar and Granoff that begins on page 891.
Model by which the classical and alternative complement pathways mediate SBA or OPA killing of meningococci. Blocking cleavage of C5 or C7 in the terminal complement pathway prevents formation of MAC, which is required for complement-mediated SBA. In the absence of MAC, anti-meningococcal antibodies may offer protection by eliciting antibody-Fc–mediated and/or C3b/iC3b complement receptor–mediated OPA. However, complement receptor–mediated phagocytic uptake as well as phagocytic cell chemotaxis is promoted by C5a, a split product of C5. Thus, blockage of release of C5a by eculizumab could abrogate this additional protective mechanism. See Figure 1 in the article by Konar and Granoff that begins on page 891.
Dysregulated and excessive complement activity causes a number of life-threatening disorders, including paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). Eculizumab is a humanized monoclonal antibody that binds to C5 and inhibits the formation of the complement terminal complex (membrane attack complex [MAC]). Eculizumab was licensed in 2007 and has proved effective in improving both PNH and aHUS, in some cases to a remarkable degree, with significant improvement in survival and quality of life.
Children born with genetic deficiencies that prevent formation of MAC are particularly susceptible to meningococcal infection. The incidence of meningococcal infections in patients with congenital complete deficiency in terminal complement factors is 0.5% per year, a relative risk of 5000 compared with the normal population.2 The eculizumab package insert contains a black-box warning from the US Food and Drug Administration, recommending immunization against meningococcal disease 2 weeks before initiation of complement blockade therapy. Antibiotic prophylaxis is not recommended. Despite this precaution, it is apparent that a proportion of persons treated with eculizumab will have breakthrough meningococcal infection despite immunization and, in some cases, despite both immunization and antibiotic prophylaxis.3-5
Konar and Granoff examined the effect of eculizumab on killing of meningococci by whole blood from healthy adults immunized with meningococcal serogroup A, C, W, Y, and serogroup B vaccines using an ex vivo whole-blood model of meningococcal sepsis. This elegant model is particularly useful because it incorporates 2 important mechanisms by which serum antibodies confer protection against meningococcal disease: both serum bactericidal activity (SBA) and opsonophagocytic (OPA). The data show poor OPA killing of meningococci by serum from immunized individuals in the presence of eculizumab. Meningococci were not killed by blood containing eculizumab because release of C5a, a C5 split product needed for upregulation of phagocytosis, was inhibited. In contrast, meningococcal killing was relatively preserved in the presence of an inhibitor of complement factor D (ACH-4471), a regulator of the alternative pathway of complement activation that is in early-phase trials for treatment of PNH. These new data (summarized; see figure) offer an explanation for the breakthrough of meningococcal infection in seemingly well-immunized persons receiving eculizumab.
These new data have a number of important clinical implications. First, it is clear that immunization alone should not be relied upon to protect persons receiving eculizumab. Additional antibiotic prophylaxis would seem wise, together with a very high level of suspicion for meningococcal disease with any febrile illness in any person treated with eculizumab. Consideration might be given to modification of the black-box warning to alert prescribers to this need, and active dissemination of the data will help avoid unnecessary infections. Eculizumab is being used increasingly to treat microangiopathy in the setting of hematopoietic stem cell transplant (HSCT).6,7 HSCT recipients have severely reduced B-cell function and are unlikely to respond to immunization, although some centers report administering the immunization anyway because of the black-box warning. Our own clinical practice in HSCT recipients is to use antibiotic prophylaxis for the duration of complement blockade (determined by return of CH50 levels to normal), and we have treated >70 patients without meningococcemia.8
Our antibiotic prophylaxis strategy may have been particularly successful because the duration of treatment of our patients was finite, and the patients were under close medical observation until complement blockade resolved. Many persons with PNH or aHUS receive eculizumab prophylaxis indefinitely, making lapses in compliance with long-term prophylaxis likely. The optimal duration of prophylactic complement blockade for persons with aHUS remains undetermined. When complement blockade first became available, there was reasonable concern regarding relapse, and long-term therapy was initiated for many or most of those treated. Recent guidelines have proposed that constant blockade may not be necessary for persons at low risk for recurrence, for example, persons with specific low-risk complement gene variants or with low levels of factor H antibodies.9 The continued risk of meningococcal infection, in addition to the considerable cost of therapy, and the burden of chronic IV drug administration, add additional incentive to explore the cessation of therapy in persons with aHUS. Brodsky and colleagues recently reported successful cessation of therapy in a majority of a cohort of 17 adults with aHUS, with a modest relapse rate, and retrieval of renal function with reinitiation of complement blockade in relapsed cases.10 It should be noted, however, that follow-up is relatively short and additional events may occur with longer follow-up.
Konar and Granoff also tested the effect of investigational factor D inhibitor ACH-4471 (which acts on the alternative complement pathway, upstream from C5 and C5a) on bacterial killing. Factor D inhibition had less effect on meningococcal killing, supporting their data that the impaired meningococcal killing seen with eculizumab in the presence of immune serum is due to reduced phagocyte activation from lower levels of C5a, a potent chemoattractant and activator. Currently, a number of different complement blockers, in addition to the factor D inhibitor, are under investigation, including an engineered complement receptor 2/factor H fusion protein TT30, members of the peptide C3 inhibitor compstatin family, and a C1 esterase inhibitor, C1INH. These newer molecules may be effective alone or in combination and could ameliorate the risk of meningococcal disease in the future.
Conflict-of-interest disclosure: The author declares no competing financial interests.