In this issue of Blood, Mortera-Blanco et al provide definitive evidence of the origin of myelodysplastic syndrome (MDS) with ring sideroblasts (RS) by tracing mutations in the splicing factor SF3B1 back to phenotypic hematopoietic stem cells (HSC) with multilineage potential. This study shows not only that SF3B1 mutations are early events in MDS and identifiable in myeloid lineage but also that SF3B1 lesions are detectable in pro-B cells. The authors also present a patient-derived mouse xenograft model that recapitulates MDS with RS phenotype from multipotent SF3B1-mutant HSCs derived from MDS-RS patients.1
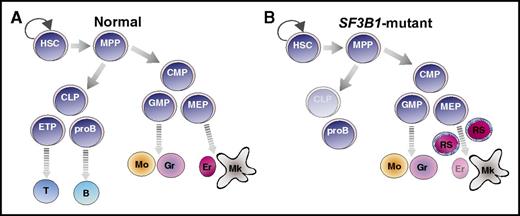
SF3B1 mutations in the hematopoietic hierarchy of MDS-RS. Cartoon illustration of normal (A) and SF3B1-mutant (B) MDS-RS hematopoietic hierarchy. SF3B1 mutations are detected in HSC, myeloid progenitors, mature myeloid types, and pro-B cells of MDS-RS patients (B). HSCs, but not progenitors, propagate the disease. B, B-cell; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; Er, erythrocyte; ETP, early T-cell progenitor; GMP, granulocyte-monocyte progenitor; Gr, granulocyte; MEP, megakaryocyte-erythrocyte progenitor; Mk, mature megakaryocyte; Mo, monocyte; MPP, multipotent progenitor; proB, pro-B cell; T, T cell.
SF3B1 mutations in the hematopoietic hierarchy of MDS-RS. Cartoon illustration of normal (A) and SF3B1-mutant (B) MDS-RS hematopoietic hierarchy. SF3B1 mutations are detected in HSC, myeloid progenitors, mature myeloid types, and pro-B cells of MDS-RS patients (B). HSCs, but not progenitors, propagate the disease. B, B-cell; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; Er, erythrocyte; ETP, early T-cell progenitor; GMP, granulocyte-monocyte progenitor; Gr, granulocyte; MEP, megakaryocyte-erythrocyte progenitor; Mk, mature megakaryocyte; Mo, monocyte; MPP, multipotent progenitor; proB, pro-B cell; T, T cell.
MDS comprises a diverse group of chronic clonal myeloid disorders characterized by cytopenia and dysplasia in one or more myeloid lineages (compare figure panel A to panel B). MDS is caused by chromosomal alterations, such as 5q deletions and monosomy 7, or frequent recurring somatic mutations in epigenetic, splicing, and transcription factors, including TET2, ASXL1, RUNX1, SRSF2, and SF3B1. SF3B1 is an essential spliceosomal protein component of the U2 small nuclear ribonucleic protein (snRNP) complex. Mutations in SF3B1 confer defective splicing and are observed in a variety of hematologic neoplasms, including chronic lymphocytic leukemia (CLL), MDS, acute myeloid leukemia (AML), and other tumor types such as melanoma and breast cancer. In particular, SF3B1 mutations are heterozygous, are mutually exclusive from mutations in other splicing factors, are frequent in MDS with RS (>80%), and are predictive of the MDS-RS subtype.2,3 MDS-RS is a form of MDS marked by accumulation of abnormal erythroid precursors with iron-filled mitochondria in a ringed pattern around the nucleus.
Mortera-Blanco et al present data that SF3B1 mutations induce myeloid lineage–restricted clonal hematopoiesis in patients with MDS-RS. This group and others have identified immunophenotypic HSCs as the originating MDS clone across many different genetic subsets, including those with SF3B1 mutations.4,5 Whereas human MDS-RS HSCs were known to produce both myeloid and lymphoid progeny in xenotransplants,4 it was thought that SF3B1 mutations did not persist in lymphoid populations on the basis of sequencing studies of MDS patient T cells.2,6 Mortera-Blanco et al confirm that T cells do not carry SF3B1 mutation in MDS-RS patients; however, they measured an increased frequency of pro-B cells in patients. In contrast to prior studies that identify SF3B1 mutations exclusively in the MDS-RS HSCs and downstream myeloid cells, Mortera-Blanco and colleagues demonstrate that MDS-RS patients’ pro-B cells also maintain SF3B1 mutations (figure panel B). Although the pathologic and therapeutic consequences of SF3B1 mutant pro-B cells remains to be explored in the context of MDS, the authors also report detection of SF3B1 mutant pro-B cells, as well as myeloid cell types, arising from MDS-RS HSCs upon transplantation in immune compromised mice. This provides a novel model system in which to study SF3B1-mutant pro-B cells in vivo.
Murine genetic models of Sf3b1 haploinsufficiency demonstrate inconsistencies in development of MDS and RS phenotypes.7,8 K700E is the most common mutational hotspot in SF3B1 in MDS-RS patients, which is thought to destabilize the U2 snRNP spliceosome. In contrast to genetic deletion, knock-in of K700E into the mouse Sf3b1 locus results in defective erythroid development and anemia, but the consequences of this mutation on HSC function in mice are less clear.9,10 Mortera-Blanco and colleagues demonstrate that transplantation of SF3B1-mutant HSCs into immune-compromised mice results in myeloid dysplasia with SF3B1 mutations maintained in myeloid (and pro-B) cell types. Importantly, these mice also develop the characteristic ring sideroblast phenotype (figure panel B). This study sets precedent for the closest recapitulation of human MDS-RS in an in vivo model to date.
This study reveals novel biology of SF3B1-mutant MDS-RS, with the identification of hidden SF3B1-mutant clones in nonmyeloid hematopoietic lineages. Although SF3B1 mutations are found in CLL, a B-cell malignancy, in MDS-RS the SF3B1 mutation is not carried beyond the pro-B stage. This suggests that other mutations may be necessary to provide a survival or growth advantage to lymphoid cells carrying SF3B1 mutations. Given that MDS is a chronic disease that can progress to AML, this new understanding of the cellular origin of MDS, in conjunction with the novel xenograft model of MDS-RS, may inform future studies aimed at identifying new therapeutic candidates to combat disease progression.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal