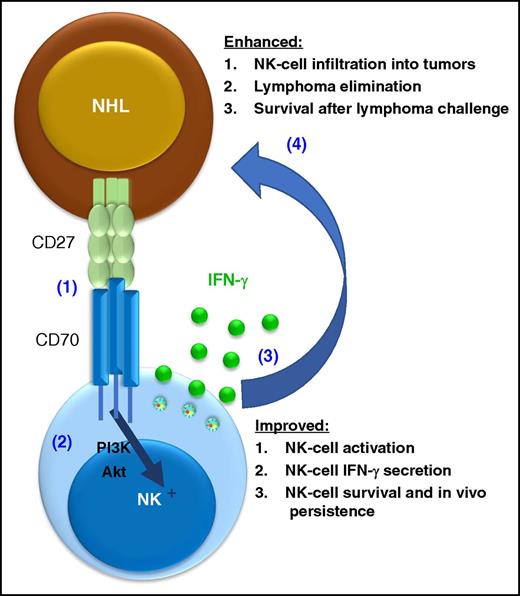
In this issue of Blood, Al Sayed et al demonstrate a critical role for CD70 signaling in natural killer (NK)–cell elimination of lymphoma, providing the pre-clinical groundwork for a new coactivating NK-cell immunotherapy strategy.1
CD70 signaling enhances NK-cell antilymphoma responses. CD70 is triggered by CD27 expressed on lymphoma cells (1), resulting in CD70-transduced phosphatidylinositol 3-kinase (PI3K)/Akt pathway, resulting NK-cell activation (2). This interaction results in NK cell activation and multiple functional effects (3), which contribute to the protection of the host against lymphoma (4).
CD70 signaling enhances NK-cell antilymphoma responses. CD70 is triggered by CD27 expressed on lymphoma cells (1), resulting in CD70-transduced phosphatidylinositol 3-kinase (PI3K)/Akt pathway, resulting NK-cell activation (2). This interaction results in NK cell activation and multiple functional effects (3), which contribute to the protection of the host against lymphoma (4).
NK cells are innate lymphoid cells specialized for host protection against viral infections that also mediate potent antitumor responses.2 However, the receptors used by NK cells to “see” and effectively respond to different types of blood cancer, including lymphoma, remain an open and important question in the field. This report sheds light on the role of CD70, an understudied receptor in the tumor necrosis factor receptor (TNFR) superfamily, to activate NK cells to respond to CD27+ lymphoma targets (see figure).1
A key player in the NK-cell response to lymphoma is CD16a (FcγRIIIa), an activation receptor that binds to the constant region (Fc) of antibodies and triggers antibody-dependent cellular cytotoxicity (ADCC). Activating NK cells via this receptor has been employed for decades in lymphoma therapy (eg, by the anti-CD20 monoclonal antibody [mAb] rituximab), with new flavors, including ADCC-optimized Fc regions and anti-CD16a bispecific killer cell engagers.3 Nonetheless, NK-cell recognition of a target involves far more than the activation of a single receptor; NK cells integrate signals from numerous inhibitory and activating receptors to “see” targets and decide whether to respond and kill a target or simply move on.4 For example, some NK cells express inhibitory killer cell immunoglobulin receptors, which turn off NK cells upon encountering their cognate HLA class I ligand. Blockade of this constitutive NK-cell checkpoint results in enhanced responses to lymphoma (including those directed by anti-CD20 mAbs),5 while increased HLA class I expression can result in lymphoma resistance to NK-cell attack.6 Such anti-CD20 mAb-directed responses can also be modulated by cytokine receptors (eg, IL-15R),7 and agonist mAbs to induced activating receptors (eg, CD137/4-1BB).8 Given the large number of NK-cell–activating receptors now appreciated, clearly linking one activating receptor to the antilymphoma responses is of translational value. Here, the authors identify CD70 as one such NK-cell–activating receptor.
In the article, several model systems are used to test the importance of CD70 for NK-cell lymphoma surveillance.1 The biology of CD70 is quite complex, and it was first described as the ligand for CD27, another TNFR superfamily member that is widely expressed on B-cell non-Hodgkin lymphoma.9 Since both CD27 and CD70 mediate signals after ligation and are expressed on multiple cell types, including T cells, B cells, and NK cells, the authors used an elegant reductionist approach to test the idea that CD70 recognition of CD27 results in augmented NK cell responses that are relevant for lymphoma elimination. A CD27-truncated receptor was used that binds CD70, triggering a response in the NK cells but delivering no CD27-based signal to the expressing cell. Expression of the truncated CD27 resulted in a marked decrease in tumor burden in both fibrosarcoma and Eμ-myc–induced lymphoma models in vivo. Importantly, this effect was clearly dependent on NK cells, since elimination of NK cells reverted the growth kinetics of CD27-truncated expressing to that of CD27-negative tumor cells. Similarly, this effect was directly related to CD70, since blockade of CD70 using antibody or genetic deletion (CD70−/−) also abrogated the antitumor response. Such loss-of-function experiments are important to demonstrate the link between CD70, NK-cell activation, and tumor elimination. Moreover, the authors provided direct evidence for a CD27-induced NK cells response: expression of the CD27-truncated protein resulted in NK-cell accumulation in tumors and enhanced interferon-γ (IFN-γ) responses by NK cells isolated from the tumor microenvironment. This is an important finding, since NK cells isolated from the blood or noninvolved lymphoid tissues lacked the enhanced responses found at in those at the site of the tumor. Typically, robust NK cell activation requires engagement of multiple activating receptors. The authors experimentally address this point by showing that CD70 cooperated with other activating rectors to enhance IFN-γ production by NK cells in vitro. From a mechanistic standpoint, ligation of CD70 on NK cells resulted in increased Akt signaling, a well-known activation pathway for NK cells. Identification of a key signaling pathway can inform the testing of complementary activating receptor agonists, cytokine receptors, or inhibitory receptor blockade to further optimize NK-cell activation and antitumor responses. To highlight the potential relevance to human disease, the authors employed a CD27+ acute lymphoblastic leukemia xenograft model and demonstrated CD70-dependent activation and persistence of adoptively transferred human NK cells. In a hypothesis-generating analysis, the authors also used public gene expression data and report univariate correlations between higher expression of CD27, CD56, and NKG2D (an NK-cell–activating receptor) and improved survival in lymphoma patients. Collectively, this study provides comprehensive data supporting CD70 as an important NK-cell–activating receptor that augments antilymphoma responses.
What does this mean for physicians who treat patients with lymphoma or lymphoma researchers developing new therapies? The findings of this study provide a clear preclinical rationale to test the therapeutic strategy of CD70 coactivation to augment NK-cell antilymphoma responses. While this report is convincing, it will be important to confirm the ability of CD70 to activate NK-cell responses in the presence of a complete immune system and with physiologic expression of its ligand, CD27. In addition, the expression of CD70 and CD27 on other immune cell types (CD4 and CD8 T cells, B cells, and dendritic cells) will need to be considered.9 Regardless, translation will also require a cautious and thoughtful approach, since CD70 is also expressed (albeit variably) on most types of lymphoma, and ligation has resulted in activation and proliferation of malignant B cells in vitro. Indeed, for these reasons, CD70 has been identified as a therapeutic antibody target being investigated in hematologic malignancies. This concern could be addressed by restricting the testing of CD70 agonists to CD70-negative lymphomas or potentially using trispecific proteins that coordinately engage CD70 and an CD16a (or another NK-cell–selective activating receptor), while the third scFv directs to the lymphoma.
Conflict-of-interest disclosure: The author declares no competing financial interests.