In this issue of Blood, Draper et al demonstrate that 2 isoforms of the transcription factor RUNX1, which are generated by alternate promoter usage, play distinct functional roles during megakaryopoiesis.1
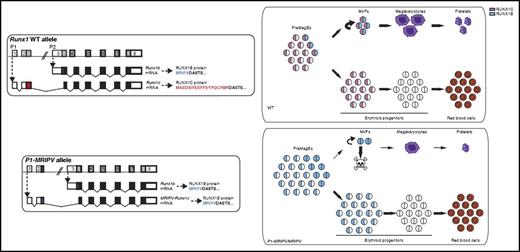
The wild-type (WT) RUNX1 genomic locus produces messenger RNA (mRNA) transcripts from either the P1 or P2 promoter generating RUNX1c or RUNX1b protein isoform, respectively. RUNX1c is expressed in preMegE progenitor cells, MkPs, and early erythroid progenitor cells, whereas RUNX1b is expressed in PreMegE cells as they commit to MkPs and during Mk maturation. Draper et al engineered the P1-MRIPV allele by replacing DNA sequences in exon2 that encode the normal MASDSIFESFPSYPQCFMR amino acid sequence (unique to RUNX1c) with those encoding MRIPV (unique to RUNX1b). Homozygous P1-MRIPV knock-in mice, which lack expression of all RUNX1c, have impaired survival of MkPs, reduced megakaryocyte output, and enhanced erythroid output. The MkPs that survive have normal terminal megakaryocyte maturation. The figure has been adapted from Figures 1 and 7 in the article by Draper et al that begins on page 271.
The wild-type (WT) RUNX1 genomic locus produces messenger RNA (mRNA) transcripts from either the P1 or P2 promoter generating RUNX1c or RUNX1b protein isoform, respectively. RUNX1c is expressed in preMegE progenitor cells, MkPs, and early erythroid progenitor cells, whereas RUNX1b is expressed in PreMegE cells as they commit to MkPs and during Mk maturation. Draper et al engineered the P1-MRIPV allele by replacing DNA sequences in exon2 that encode the normal MASDSIFESFPSYPQCFMR amino acid sequence (unique to RUNX1c) with those encoding MRIPV (unique to RUNX1b). Homozygous P1-MRIPV knock-in mice, which lack expression of all RUNX1c, have impaired survival of MkPs, reduced megakaryocyte output, and enhanced erythroid output. The MkPs that survive have normal terminal megakaryocyte maturation. The figure has been adapted from Figures 1 and 7 in the article by Draper et al that begins on page 271.
RUNX1 is a key hematopoietic and endothelial transcription factor that plays critical roles in definitive hematopoietic stem-cell biogenesis and the differentiation of essentially all major hematopoietic lineages except the erythroid lineage. It is particularly important during megakaryopoiesis, where it directly controls the expression of genes involved in megakaryocyte survival and maturation, endomitosis, and platelet formation. Germ line loss-of-function or dominant-negative acting mutations cause an autosomal-dominant syndrome of impaired megakaryocyte maturation, thrombocytopenia, platelet dysfunction, and leukemia predisposition. The RUNX1 gene is also a frequent target of somatic chromosomal rearrangements and point mutations in leukemia and myelodysplastic syndrome.
RUNX1 is normally transcribed from 2 separate promoters: a distal promoter designated P1 and a proximal promoter designated P2.2 Transcription from these 2 promoters generates slightly different protein products. The P1 transcript produces a protein (called RUNX1c) that is 14 amino acids longer than that produced from the P2 transcript and contains the specific amino acid sequence MASDSIFESFPSYPQCFMR at its amino terminus. The P2 transcript produces a protein (called RUNX1b) that contains the alternate amino terminal sequence MRIPV. The remainder of the protein is identical between the 2 isoforms. A key question in the field has been whether the 2 isoforms play distinct functional roles in hematopoiesis.
Draper et al3 previously showed that RUNX1c is the predominantly expressed isoform during adult hematopoiesis. RUNX1b expression is much more limited, but it is significantly upregulated as bipotent premegakaryocyte/erythroid progenitor cells (preMegEs) commit to the megakaryocyte lineage. In the current study, Draper et al take a novel approach to test the functional requirements for each isoform by generating knock-in mice in which the sequence that normally encodes the unique RUNX1c amino terminus is replaced by that of RUNX1b. The P1 transcribed 5′UTR region is retained in this knock-in allele, preserving the stability and translation efficiency of the normally expressed RUNX1c transcript. Homozygous P1-MRIPV knock-in mice therefore express RUNX1b exclusively and do so with an expression pattern that mimics both endogenous RUNX1c and RUNX1b. Importantly, the authors demonstrate that total RUNX1 protein levels are equivalent in the homozygous knock-in and wild-type mice.
The homozygous knock-in mice have modest thrombocytopenia at steady state. However, further analysis revealed that they have a significantly reduced number of megakaryocyte progenitor cells (MkPs) and colony-forming unit megakaryocyte cells but increased numbers of burst-forming unit erythroid cells and colony-forming unit erythroid cells (see figure). Likewise, ex vivo culture of purified PreMegEs shows significantly reduced megakaryocytic output with concomitant increased erythroid output. There is also increased late apoptosis of MkPs. However, the mutant MkPs that survive undergo full terminal megakaryocyte maturation. This latter finding contrasts with what is observed in full RUNX1 hematopoietic knockout mice, in which both megakaryocyte lineage commitment and maturation are severely impaired.4-6 Collectively, these findings provide strong evidence for a selective requirement of the RUNX1c isoform in cell-fate choice of preMegEs for the megakaryocyte lineage and for survival of early committed MkPs.
This is one of the first studies to provide in vivo evidence for the nonequivalency of RUNX1b and RUNX1c during native adult hematopoiesis and strongly suggests that these differences relate to the altered amino terminal amino acid sequences of the 2 isoforms. It will be of interest to further understand the mechanisms that underlie the functional differences of these 2 isoforms. Prior work has demonstrated that RUNX1c has higher DNA binding affinity than RUNX1b.7 It is therefore possible that differences in chromatin occupancy site usage could account for the different behaviors. It is also possible that differences in protein-protein interactions, conformation, stability, and/or cell localization could be involved.
The mechanisms that mediate cell-fate choice of preMegEs for the megakaryocyte versus erythroid lineage are just beginning to be elucidated. Prior work has shown that the major megakaryocyte transcription factor FLI1 cross-antagonizes the key erythroid-specific transcription factor KLF1 in this decision and that FLI1 physically and functionally cooperates with RUNX1.8,9 Recent work by Kuvardina et al10 showed that RUNX1 directly represses transcription of the KLF1 gene. Given the findings of Draper et al in the current study, it would be of interest to determine if RUNX1c, but not RUNX1b, is involved in this repressive event.
Draper et al previously showed that RUNX1 expression switches from being predominantly P1 to P2 directed during megakaryocyte maturation. In the current study, they demonstrate that RUNX1b is sufficient to support normal megakaryocyte maturation beyond the MkP stage. It would therefore be of interest to perform the converse experiment to that described in the current study, in which the RUNX1c sequences would be substituted for those of RUNX1b at the P2 promoter. This would determine whether RUNX1b is not only sufficient but also selectively required for terminal megakaryocyte maturation.
The structure of the RUNX1 gene locus, with its dual promoters and isoform-specific transcript products, is highly evolutionarily conserved and also occurs in the 2 other known mammalian RUNX family genes, RUNX2 and RUNX3.2 These factors play broad roles in normal development. It will be of interest to apply the experimental approach of Draper et al to these other family members and in other cellular contexts to determine whether the functional significance of RUNX alternate promoter usage and isoform specificity is broadly applicable. It also points to the importance of further understanding the factors and signaling events that dictate P1 vs P2 promoter usage.
There is considerable interest in generating platelets in vitro from human-induced pluripotent stem cells for ultimate transfusion purposes. However, the extremely low efficiency of platelet generation with current culture systems remains a major obstacle in this field. The work of Draper et al suggests that manipulation of specific RUNX1 isoforms during ex vivo culture could potentially be used to enhance platelet production via skewing of progenitor cell-fate choice toward the megakaryocyte lineage, improving MkP survival and supporting terminal megakaryocyte maturation.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal