Abstract
In chronic lymphocytic leukemia (CLL) patients with mutated IGHV, 3 recent studies have demonstrated prolonged progression-free survival (PFS) after treatment with fludarabine-cyclophosphamide-rituximab (FCR) chemoimmunotherapy. We performed a systematic review to assess the benefit of FCR for patients with CLL and identified 5 randomized trials that met our inclusion criteria. FCR improved complete remission, PFS and overall survival vs the comparator; median PFS was not reached in the subgroup of CLL patients with mutated IGHV.
Case presentation
A 62-year-old woman with no significant past medical history presented 6 years ago with a white blood cell count (WBC) of 20.5 × 109/L (predominantly lymphocytes) and was found to have a monoclonal λ-expressing B-cell population of 12.0 × 109/L, coexpressing dim CD5 and dim CD20, CD19, and CD23, consistent with chronic lymphocytic leukemia (CLL). Fluorescence in situ hybridization showed 13q deletion in 84.5% of cells; a stimulated karyotype was normal. Immunoglobulin heavy-chain testing demonstrated clonal rearrangement of IGHV4-59, which was 95.2% similar to its closest germ line match, consistent with mutated immunoglobulin heavy-chain variable region (IGHV). Despite these favorable markers, her WBC has risen steadily (now 185.0 × 109/L), with worsening anemia (hemoglobin, 9 g/dL) and thrombocytopenia (platelets, 77.0 × 109/L). She now meets International Workshop on Chronic Lymphocytic Leukemia criteria for initiation of therapy. What is your therapy of choice for this patient?
Introduction
Over the last several decades, chemoimmunotherapy for chronic lymphocytic leukemia (CLL) has evolved significantly from single-agent chlorambucil, to fludarabine,1 to fludarabine and cyclophosphamide (FC),2,3 and, ultimately, to fludarabine, cyclophosphamide, and rituximab4 (FCR). Each step increased the likelihood of complete remission (CR) and improved progression-free survival (PFS). It was not until the advent of FCR, however, that an improvement in overall survival (OS) was also seen, as demonstrated at the first report with 3-year follow-up of the CLL8 trial of the German CLL Study Group5 (GCLLSG). Given the increasing use of bendamustine and rituximab (BR) in the community, the GCLLSG performed a second more recent randomized trial, CLL10, comparing FCR to BR, which demonstrated that FCR resulted in better CR, minimal residual disease (MRD) negativity, and PFS compared with BR, albeit at the cost of increased infectious toxicity, particularly in older patients.6 Recently, targeted therapies have emerged, led by ibrutinib, which demonstrated PFS and OS benefit compared with single-agent chlorambucil in frontline therapy of patients over 65 years without 17p deletion.7
FCR was observed early on to result in frequent CRs and long remissions, including some that were negative for MRD as measured by the internationally standardized 4-color flow cytometry approach.8,9 It was not initially suspected, however, that FCR might result in a plateau on the PFS curve, with extremely long remissions, until a 6-year follow-up report of the first cohort of 300 patients treated with upfront FCR,10 which showed a 51% 6-year failure-free survival. These excellent results heralded a subsequent report with 12.8 years follow-up, in which overall PFS was 30.9% and median PFS was 6.4 years.11 Mutated immunoglobulin heavy-chain variable region (IGHV) proved a strong predictor of long-term PFS, with 53.9% of patients still in remission at 12.8 years and no relapses among 42 patients beyond 10.4 years follow-up, suggesting a long-term plateau. Furthermore, among the 51% of mutated IGHV patients who achieved posttreatment MRD negativity, PFS was 79.8% at 12.8 years follow-up. In contrast, only 8.7% of those with unmutated IGHV were in ongoing remission at 12.8 years. Similar trends in IGHV-mutated patients have been observed with long-term follow-up of the CLL8 trial, now at 5.9 years,12 showing median PFS of 56.8 months for FCR vs 32.9 months for FC. Among patients with mutated IGHV, the median PFS was not reached compared with 42 months with FC (hazard ratio [HR], 0.47). A retrospective analysis of an Italian population treated with FCR found that among the 28% of patients with mutated IGHV and without higher risk deletions of 11q or 17p,13 71% were free of progression at 4 years, and at that point had a life expectancy comparable to the age- and sex-matched population without CLL.14
The impact of cytogenetics as a long-term predictor of PFS after FCR has been less well evaluated, as fluorescence in situ hybridization (FISH) was not yet routine at the time of the first MD Anderson FCR study.11 However, del(17p) by karyotype in the MD Anderson study, and by FISH in CLL8, were associated with markedly worse outcomes for both PFS and OS.11,12 Interestingly, del(11q) was not associated with a lower likelihood of MRD negativity or worse PFS in a landmark analysis of early MD Anderson data focused on MRD negativity9 ; it was, however, an independent predictor of decreased PFS in the German data, which had longer follow-up. The Italian study used recursive partitioning to identify the combined features of mutated IGHV and absence of 11q and 17p deletions as low risk.14 Most patients with del(11q) and del(17p) are unmutated, and so data are sparse on the impact of these abnormalities within the mutated IGHV subset. This information was reported separately in the German data and showed that del(17p) but not del(11q) remained adverse within the mutated IGHV subgroup.12
Systematic review
To evaluate the strength of the evidence supporting long-term PFS after FCR, and in what patient subgroups, we conducted a systematic search and meta-analysis of clinical studies investigating the efficacy of FCR in patients with untreated CLL. Only randomized controlled trials comparing FCR to other treatment regimens were included. The methodology and reporting of the results used in this study were based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) recommendations15 (see supplemental Methods, available on the Blood Web site, for details of the search strategy). We conducted a pairwise analysis comparing FCR vs control using the DerSimonian and Laird method with the estimate of heterogeneity from the Mantel-Haenszel model.16 With respect to time-to-event outcome, we estimated the individual patient level data (IPD) using the algorithm described by Guyot et al.17 We conducted a 2-stage IPD meta-analysis for pooled HRs with corresponding confidence interval (CI).18 Estimated PFS and OS were calculated.
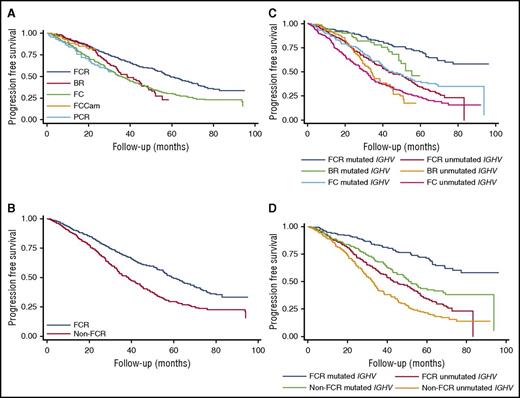
The results of the literature search are illustrated in supplemental Figure 1. Following removal of duplicates, the literature search yielded 2822 records for screening, of which 137 were full-text articles assessed for eligibility. Five randomized trials were ultimately included in the quantitative meta-analysis, all of which compared FCR to a different comparator (supplemental Table 1).6,12,19-21 This analysis showed no significant difference in the overall response rate between FCR and the pooled comparator arms (risk ratio [RR], 1.04; 95% CI, 0.96-1.13; P = .32; I2 = 83.8%; supplemental Figure 2), whereas the likelihood of achieving CR was increased by FCR overall (RR, 1.50; 95% CI, 1.07-2.10; P = .02; I2 = 83.2%; supplemental Figure 3), and in both mutated and unmutated IGHV subgroups in the 3 studies with available data (supplemental Figure 4). Only 3 studies reported evaluable MRD data, which showed an increased likelihood of achieving MRD negativity with FCR compared with the combination of BR/fludarabine, cyclophosphamide, mitoxantrone, and rituximab (FCM-R)/fludarabine, cyclophosphamide, and alemtuzumab (FCCam) (RR, 1.21; 95% CI, 1.06-1.38; P = .005; I2 = 0%; supplemental Figure 5). No significant difference was seen among trials in total grade 3-4 adverse events, in hematologic or infectious grade 3-4 adverse events, or in second malignancies (supplemental Figure 6). Comparison of PFS in these trials demonstrates that FCR was significantly superior to BR and FC but not to FCCam and pentostatin, cyclophosphamide, and rituximab (PCR) (Table 1; Figure 1A), with a combined comparator HR for progression of 1.65 (95% CI, 1.42-1.91; P < .001; I2 = 0%; Table 1; Figure 1B; supplemental Figure 7). Comparison of OS showed that FCR was statistically superior to FC, but not to BR, FCCam, or PCR (supplemental Figures 8-9), although FCR did improve OS compared with the combined comparator (HR, 1.39; 95% CI, 1.12-1.72; P = .003; I2 = 0%; supplemental Table 2; supplemental Figure 9).
PFS: individual patient data estimation. (A) PFS comparing FCR to non-FCR regimens. (B) PFS for FCR vs combined comparator. (C) PFS for FCR vs non-FCR regimens, by IGHV mutational status. (D) PFS for FCR vs combined comparator, by IGHV mutational status.
PFS: individual patient data estimation. (A) PFS comparing FCR to non-FCR regimens. (B) PFS for FCR vs combined comparator. (C) PFS for FCR vs non-FCR regimens, by IGHV mutational status. (D) PFS for FCR vs combined comparator, by IGHV mutational status.
Data for PFS and OS by IGHV mutation status were limited to 2 studies, CLL8 and CLL10. In this analysis, the only subgroup in which a median PFS was not yet reached was the IGHV-mutated patients treated with FCR, and these patients had statistically improved PFS compared with all other IGHV and treatment subgroups (Table 1, “Classified by regimen and IGHV mutation status” section; Figure 1C). The IPD meta-analysis showed that risk of progression was greater in either patients who had unmutated IGHV treated with FCR or mutated IGHV treated with comparator (Table 1, “Classified by IGHV mutation status” section; Figure 1D). Only 1 study reported PFS and OS subgroups by cytogenetics so a meta-analysis was not performed. The quality of the evidence is summarized in supplemental Table 3.
Discussion
Three recent studies, only 1 of which was a randomized trial, have reported long-term PFS, particularly in IGHV-mutated CLL patients treated with FCR.11,12,14 We have performed a systematic review of the data that support the benefit of FCR compared with other chemoimmunotherapy regimens in CLL, and identified 5 randomized trials for inclusion. The combined analysis demonstrates benefits to both PFS and OS with FCR compared with comparator and adds to the body of data demonstrating that, in fit CLL patients, FCR is the most effective chemoimmunotherapy regimen. BR is widely used but has not demonstrated the durability of remission seen with FCR and should not be considered an equivalent substitute for FCR in CLL. That being said, many CLL patients are not candidates for FCR therapy due to comorbid medical conditions and/or renal dysfunction, and for these patients BR or other less-intense therapies are an option.
Evaluation of the impact of IGHV mutational status on survival outcomes in this systematic review is unfortunately more limited, as none of the randomized trials was prospectively stratified to evaluate the impact of this prognostic marker. Only 2, both from the GCLLSG, reported adequate data for this subgroup analysis, 1 of which had individually reported the same finding. Thus, our meta-analysis is limited and much of the data supporting the prolonged benefit of FCR in the IGHV-mutated subgroup is based on the observational studies. Nonetheless, the randomized data are supportive of prolonged PFS particularly with FCR in the IGHV-mutated subgroup, the only group in which the median has not been reached (median follow-up ranged from 31 to 71 months). Interestingly, the comparator results in the IGHV-mutated subgroup were superior to FCR in the IGHV-unmutated group, underscoring the singular importance of IGHV in the setting of chemoimmunotherapy treatment.
The data are even more limited as to the impact of cytogenetics, in which no systematic review could be performed for PFS or OS due to inadequate data. The 3 original reports of long-term PFS after FCR generally agree that 17p deletion predicts poor PFS with chemoimmunotherapy, but the data beyond that are incomplete. For example, full FISH data are not available for patients in the original MD Anderson study,11 whereas the Italian study identified a favorable subgroup with neither 17p nor 11q deletion. The long-term follow-up of CLL8 showed that 11q deletion was associated with reduced PFS in the overall population, but did not negatively impact the very favorable PFS of a mutated IGHV patient.12 Thus, only deletion 17p has been definitely shown in multiple studies to exclude patients from the potential long-term benefit of FCR.
The impact of newly identified prognostic factors on durable remissions after FCR is also unknown. For example, complex karyotype, typically defined as 3 or more abnormalities by stimulated metaphase analysis, has been reported as adverse in the setting of ibrutinib therapy,22-24 but was not evaluated in any of the FCR studies. The close association of complex karyotype with high-risk cytogenetics would urge caution, however. Recurrent somatic mutations in CLL, particularly NOTCH1 and SF3B1, have negative prognostic impact in multiple retrospective studies.25-28 In CLL8, SF3B1 mutation was associated with reduced PFS but not OS, and NOTCH1 was associated with loss of rituximab benefit.29 However, the impact, if any, of these mutations on FCR benefit in mutated IGHV patients has not to our knowledge been evaluated in any study. This question needs to be addressed, as no data are presently available to guide practice. Our view is that, for the present, given the overwhelming significance of mutated IGHV in predicting favorable PFS and OS after FCR, we would still consider patients with these markers to be eligible for FCR, particularly in the setting of mutated IGHV.
No difference in adverse events including second malignancies was identified with FCR vs comparator in our analysis. Much of the risk of second malignancies in CLL patients is disease related rather than therapy related, but the small risk of therapy-related myelodysplastic syndrome or acute myeloid leukemia after chemoimmunotherapy, recently estimated at 1.2%, remains a concern.30
CLL therapy has undergone a revolution in the last several years with the advent of the targeted small-molecule inhibitors, in particular the Bruton tyrosine kinase inhibitor ibrutinib. Ibrutinib is approved by the US Food and Drug Administration for the frontline therapy of any CLL patient and is increasingly used in the frontline setting. However, the randomized data supporting this use come from a restricted patient population over 65 years of age and without 17p deletion.7 To date, no clinical trials have been reported that enrolled the younger fit patient population on frontline ibrutinib, and younger age has been reported as a risk factor for progressive disease on ibrutinib.31 Two ongoing randomized trials of FCR vs ibrutinib-rituximab, 1 in the US Intergroup (NCT02048813) and 1 in the UK CLL group (ISRCTN01844152), will provide short-term PFS data likely in the next several years. Unfortunately, however, neither study is stratified by IGHV mutation status, and will not have follow-up to rival the current FCR studies for at least a decade. Thus, FCR will remain the standard of care for mutated IGHV patients, and ongoing clinical trials evaluating the addition of ibrutinib to FCR or FCR-like chemoimmunotherapy have achieved very high rates of MRD negativity, which will hopefully further improve outcomes in this patient subgroup.32,33
General recommendations
Three separate studies now demonstrate the potential for long-term remission among IGHV-mutated patients without deletion 17p, treated with FCR. In addition, a systematic review of randomized trial data consistently demonstrates the benefit of FCR compared with comparator, for CR (moderate-quality evidence), for PFS (moderate-quality evidence), and for OS (low-quality evidence). Given these data, together with other benefits that include its limited time duration and relatively low cost compared with ibrutinib,34 FCR should remain the standard of care for fit IGHV-mutated patients eligible for potent chemoimmunotherapy. Thus, the treatment of choice for our case patient above is FCR. Benefit of FCR, including MRD-negative CR, is also seen among IGHV-unmutated patients, but continuous relapse occurs, making it ideal to refer these patients for clinical trials.
The online version of this article contains a data supplement.
Authorship
Contribution: C.C.-A. and J.R.B. were both involved in study conception and design, data analysis, the drafting of the paper, and approval of the final manuscript.
Conflict-of-interest disclosure: J.R.B. has served as a consultant for Janssen, Pharmacyclics, AstraZeneca, Sun Pharma, Redx Pharma, Gilead, AbbVie, and Genentech/Roche. C.C.-A. declares no competing financial interests.
Correspondence: Jennifer R. Brown, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: jennifer_brown@dfci.harvard.edu.