Key Points
Ibrutinib induced a high rate of sustained responses for patients with cGVHD and inadequate response to corticosteroid-containing therapy.
This trial supported the approval of ibrutinib for treatment of adult patients with cGVHD after failure of ≥1 lines of systemic therapy.
Abstract
Chronic graft-versus-host disease (cGVHD) is a serious complication of allogeneic stem cell transplantation with few effective options available after failure of corticosteroids. B and T cells play a role in the pathophysiology of cGVHD. Ibrutinib inhibits Bruton tyrosine kinase in B cells and interleukin-2–inducible T-cell kinase in T cells. In preclinical models, ibrutinib reduced severity of cGVHD. This multicenter, open-label study evaluated the safety and efficacy of ibrutinib in patients with active cGVHD with inadequate response to corticosteroid-containing therapies. Forty-two patients who had failed 1 to 3 prior treatments received ibrutinib (420 mg) daily until cGVHD progression. The primary efficacy end point was cGVHD response based on 2005 National Institutes of Health criteria. At a median follow-up of 13.9 months, best overall response was 67%; 71% of responders showed a sustained response for ≥20 weeks. Responses were observed across involved organs evaluated. Most patients with multiple cGVHD organ involvement had a multiorgan response. Median corticosteroid dose in responders decreased from 0.29 mg/kg per day at baseline to 0.12 mg/kg per day at week 49; 5 responders discontinued corticosteroids. The most common adverse events were fatigue, diarrhea, muscle spasms, nausea, and bruising. Plasma levels of soluble factors associated with inflammation, fibrosis, and cGVHD significantly decreased over time with ibrutinib. Ibrutinib resulted in clinically meaningful responses with acceptable safety in patients with ≥1 prior treatments for cGVHD. Based on these results, ibrutinib was approved in the United States for treatment of adult patients with cGVHD after failure of 1 or more lines of systemic therapy. This trial was registered at www.clinicaltrials.gov as #NCT02195869.
Introduction
Chronic graft-versus-host disease (cGVHD) is a serious and life-threatening complication of allogeneic hematopoietic stem cell transplantation affecting 30% to 70% of patients.1 It is a leading cause of late nonrelapse mortality for transplant patients, also contributing to morbidity and a decrease in quality of life.2-5 Corticosteroids, the standard frontline treatment, are typically administered for a median of 2 to 3 years,6 leading to substantial morbidity. An effort to decrease corticosteroid doses has led to their use in combination with other immunosuppressants, such as cyclosporine, tacrolimus, and sirolimus, in frontline or second-line settings, despite a lack of clinical evidence supporting additional efficacy after combining these agents with corticosteroids.7-12 Patients who have persistent cGVHD after frontline therapy and require a change in treatment have a 2.5 times increased risk of nonrelapse mortality13 ; however, there is no standard of care or approved second-line treatment.14 An effective treatment option for patients with cGVHD that fails to respond to initial therapy remains an unmet medical need.15
Both B and T cells play critical roles in the pathogenesis of cGVHD.16-19 A lower incidence of cGVHD after in vivo T-cell depletion confirms T-cell involvement, although higher rates of infections and relapse of underlying malignancy complicate this approach.20,21 Host-reactive B cells are also associated with the development of cGVHD,18 and rituximab provides clinical benefit; however, alloreactive B cells recur after treatment discontinuation.22
Ibrutinib is a first-in-class, once-daily inhibitor of Bruton tyrosine kinase (BTK). Activation of the B-cell receptor triggers the BTK signaling pathway, which regulates B-cell survival.23 Ibrutinib also inhibits interleukin-2–inducible T-cell kinase (ITK); stimulation of ITK, mediated by phospholipase C γ (PLCγ), is involved in the selective activation of T-cell subsets that drive immune reactivity toward healthy tissues.24 In preclinical models, mice that received BTK- or ITK-deficient bone marrow transplants did not develop cGVHD, indicating that both kinases play critical roles in cGVHD pathogenesis.25 By inhibiting both BTK and ITK, ibrutinib has the potential to provide a clinical benefit for cGVHD. In preclinical models, ibrutinib delayed progression and improved clinical manifestations of cGVHD.25 In a recent analysis of patients with relapsed chronic lymphocytic leukemia after allogeneic hematopoietic stem cell transplantation, ibrutinib was tolerable and effective.26
Based on the biological rationale and compelling preclinical data, a phase 1b/2 study was designed to evaluate the safety and efficacy of ibrutinib in patients with cGVHD that has failed to respond to at least 1 systemic corticosteroid-based therapy and who needed additional treatment. Supported by the results of this trial, ibrutinib was recently approved in the United States for the treatment of adult patients with cGVHD after failure of 1 or more lines of systemic therapy.
Patients and methods
Patients
Starting on July 14, 2014, eligible patients were enrolled if they were aged ≥18 years, had steroid-dependent or -refractory cGVHD after hematopoietic stem cell transplant, and had received ≤3 prior regimens for cGVHD. Steroid-dependent disease was defined as cGVHD requiring prednisone ≥0.25 mg/kg per day for ≥12 weeks; refractory disease was defined as progressive cGVHD, despite treatment with prednisone ≥0.5 mg/kg per day for ≥4 weeks. Active cGVHD was required, and patients were to have either >25% body surface area erythematous rash or a National Institutes of Health (NIH) mouth score >4. These manifestations were selected because they were expected to respond rapidly to an effective therapy, and thus the patient could potentially avoid long-term exposure to an ineffective therapy.
Pretransplant use of ibrutinib for reasons other than cGVHD, such as for the treatment of leukemia or lymphoma, was permitted. All patients received systemic corticosteroid therapy for cGVHD prior to and during the study; concomitant use of other immunosuppressive therapies including extracorporeal photopheresis was also permitted; however, preexisting corticosteroid and immunosuppressant doses must have been stable for 14 days before initiating ibrutinib. Doses of concomitant corticosteroids and immunosuppressants could be tapered during the study as clinically indicated.
Study design and treatment
This phase 1b/2, open-label, multicenter study was designed to determine the safety and efficacy of ibrutinib in patients who failed ≥1 therapy for cGVHD. Phase 1b was conducted using a modified 3+3+3 design to evaluate the safety of daily oral ibrutinib and determine the recommended phase 2 dose (RP2D). Treatment was initiated at an ibrutinib dose of 420 mg with 6 to 27 patients being evaluated in phase 1b depending on the frequency of dose limiting toxicities (DLTs) and need for dose reductions. If unacceptably high DLTs were seen, the ibrutinib dose could be sequentially reduced to 280 mg and then 140 mg. Patients in phase 1b who did not experience a DLT were permitted to continue treatment and follow-up in phase 2 at their phase 1 dose. In phase 2, patients were treated with ibrutinib at the RP2D along with preexisting immunosuppressants for cGVHD and followed for signs of progression or improvement of cGVHD. Approximately 34 patients were to be enrolled in phase 2 with a total target enrollment of ∼40 patients in phase 1b and phase 2 who were treated at the RP2D. For this analysis, patients were followed until September 1, 2016.
The study (registered at www.clinicaltrials.gov as #NCT02195869) was approved by the institutional review board or independent ethics committee at each institution and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines. All patients provided written informed consent.
Study end points and evaluations
The primary end point for phase 1b was safety and tolerability, which included the number of DLTs occurring within the first 28 days on ibrutinib. The primary efficacy endpoint for phase 2 was the best overall cGVHD response rate, which was defined as the proportion of all patients who achieved a complete response (CR) or partial response (PR). All patients who had at least 1 response assessment were considered response-evaluable. Nonresponders were defined as those patients who had stable disease, had progressive disease, or were not evaluable. The response criteria were based on those provided by the 2005 NIH cGVHD Consensus Panel27 and subsequently modified to include the following 2 changes based on publication of the 2014 NIH response criteria28 : a change in organ score from 0 to 1 was not considered progression, and an organ was deemed nonevaluable for response when the organ response was confounded by a non-cGVHD–related factor. Under the original protocol, response assessments were conducted every 12 weeks. A protocol amendment added an additional response assessment at week 5.
Secondary efficacy end points included sustained response of ≥20 weeks, changes in corticosteroid requirement over time, and patient-reported change in the Lee cGVHD Symptom Scale. A decrease by ≥7 points was considered clinically meaningful and related to improved quality of life.29 Physicians and patients also reported overall cGVHD severity scores. Patients were evaluated for safety; adverse events (AEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Exploratory analyses included pharmacodynamic studies in which the effect of ibrutinib on immune cell signaling pathways, cytokines, chemokines, and factors that promote tissue fibrosis was evaluated. BTK and ITK occupancy was determined using a biotinylated covalent-binding probe to capture all unoccupied kinase. Pelleted flash-frozen peripheral blood mononuclear cells were resuspended in lysis buffer and split into 2 aliquots: 1 aliquot was incubated with the probe and the other aliquot was incubated with biotinylated primary ITK or BTK antibody to determine the total quantity of ITK or BTK protein in the sample. MSD streptavidin plates (Meso Scale Diagnotics, LLC) were used to separate biotin-conjugated proteins. The level of unoccupied or total ITK or BTK protein was quantified with a secondary antibody and Sulfo-tag detection antibody using an MSD S600 Instrument.
Immunoglobulin E (IgE)–induced basophil activation was assayed via flow cytometry using markers for CD63, CD123, and HLA-DR according to the validated procedures indicated in the BD FastImmune protocol (BD Biosciences). Briefly, 20 µL of stimulation buffer plus anti-human IgE was added to 100 µL of heparin blood and incubated at 37°C for 15 minutes. Degranulation was halted by transferring the reaction to ice and adding 10 µL of 20 mM EDTA. After staining with the appropriate antibody cocktail, red blood cells were lysed, and the samples were fixed in 0.5% paraformaldehyde. Samples were analyzed using a BD FACSCalibur flow cytometer (BD Biosciences).
Activation of PLCγ1-Y783 in CD4 T cells was measured using a phospho-flow analysis. Viable, cryopreserved T cells were assayed via flow cytometry using antibodies for CD4, CD3, and pPLCγ1 Tyr783 (Cell Signaling) with an anti-rabbit Alexa Fluor A488 (Molecular Probes) secondary antibody. Briefly, peripheral blood mononuclear cells were thawed, washed, and centrifugally plated onto precoated anti-CD3 (stimulated) or uncoated (unstimulated) wells of a sterile 6-well polystyrene cell culture dish for 5 minutes before cold quenching at 4°C. After staining for extracellular markers, cells were fixed with BD Cytofix and permeabilized with BD Perm Buffer III. The cells were then stained for intracellular markers, washed, and analyzed using a BD FACS Aria sorting flow cytometer.
Statistical analysis
With a sample size of at least 40 patients and assuming a best overall cGVHD response rate of ∼50%, the study was expected to have 90% power to show an efficacious treatment effect. The cGVHD response rate and its 95% exact binomial confidence interval were calculated using the exact test for binomial distribution.
All secondary end points and safety analyses were summarized using descriptive statistics, including means, standard deviations, and medians for continuous variables and proportions for discrete variables.
For biomarker analyses, levels of factors were determined at baseline and various time points after ibrutinib treatment. The values at each time point were expressed as a proportion of baseline value and depicted as a heat map.
Results
Patients
Six patients were enrolled in phase 1b at a dose of 420 mg. No DLTs were reported, and as a result, no dose reductions were necessary, and the RP2D of ibrutinib was determined to be 420 mg daily. An additional 36 patients were treated at 420 mg in phase 2 (total of 42 patients). The baseline characteristics of the patients are listed in Table 1. Patients had undergone both myeloablative and nonmyeloablative stem cell transplant for a variety of underlying malignancies (supplemental Table 1, available on the Blood Web site). As expected, mouth and skin were the most frequently involved organs, and 85% of patients showed evidence of cGVHD in ≥2 organs. The median Karnofsky Performance Status score was 80, with 60% of patients having a score between 60 and 80. Of the 42 patients, 28 had steroid-dependent cGVHD, 6 had steroid-refractory cGVHD, and 8 had a history of both steroid-dependent and -refractory disease. The concomitant immunosuppressive agents, which were continued during treatment with ibrutinib, are summarized in supplemental Table 2.
At a median follow-up of 13.9 months (range, 0.5-24.9 months), 12 patients (29%) were still receiving ibrutinib and 30 (71%) had discontinued treatment. Treatment duration ranged from 5.6 to 24.9 months for the 12 patients who continued treatment. The most common reasons for treatment discontinuation were AEs (n = 14), cGVHD progression (n = 5), or patient decision (n = 6); 2 patients discontinued after resolution of cGVHD symptoms (supplemental Table 3).
Safety
Most AEs were grade 1 or 2 (Table 2), with the most common being fatigue, diarrhea, muscle spasms, nausea, and bruising. The most common grade ≥3 AEs were pneumonia, fatigue, and diarrhea. Infectious complications of any grade were reported for 29 (69%) patients including 15 (36%) grade ≥3 events. Serious AEs are listed in supplemental Table 4. Two patients had a relapse of their underlying malignancy (acute lymphocytic leukemia [after 43 days on ibrutinib therapy] and prolymphocytic leukemia [after 308 days of ibrutinib therapy]). There were 7 deaths during the study with 2 occurring due to AEs (multilobular pneumonia and bronchopulmonary aspergillosis) while the patients were on ibrutinib; the other 5 deaths occurred in the follow-up period after the patients had discontinued ibrutinib, with 3 deaths attributed to cGVHD and 2 to unknown causes. No major hemorrhage events were observed. Atrial fibrillation (grade 3) was reported in 1 patient.
Dose reductions resulting from AEs were reported for 13 patients (31%); the most common AE leading to dose reductions was fatigue (n = 6). AEs led to treatment discontinuation in 14 patients (33%), with the most common reasons being fatigue (n = 3) and pneumonia (n = 2). The median duration of treatment was 1.8 months (range, 0.2-8.7 months) for patients who discontinued treatment due to unacceptable toxicity. For the 7 patients with progression of cGVHD, the median time to progression was 5.6 months (range, 1.7-15.7).
Efficacy
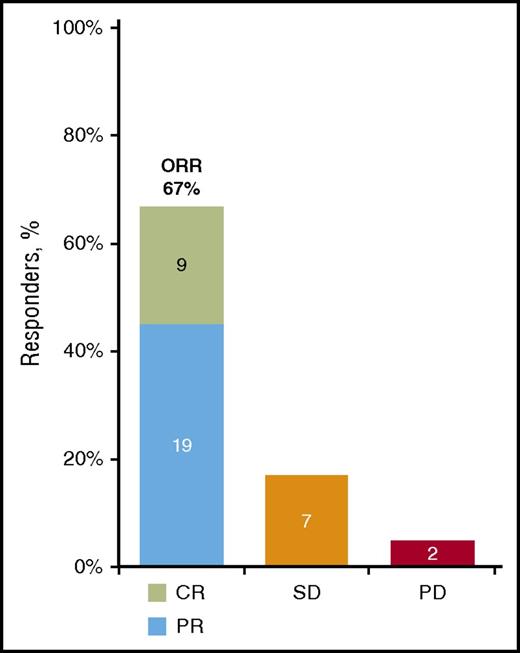
In the all treated population, the overall response rate (ORR), based on the 2005 NIH cGVHD Consensus Panel response criteria, was 67%, with a CR rate of 21% and a PR rate of 45% (Figure 1). Five patients discontinued treatment and left the study before a response assessment. Excluding these 5 patients, the ORR in the response-evaluable population was 76%. Of the 28 responders, 20 (71%) showed a sustained response for ≥20 weeks and 22 (79%) showed evidence of response at their first response assessment. For the 24 responders whose first efficacy assessment was conducted at week 13, the median time to initial response was 87 days; however, for the 4 responders who were enrolled after the protocol amendment and whose first response assessment occurred at week 5, the median time to initial response was 30 days.
Best cGVHD response. The best cGVHD response was measured based on the 2005 NIH response criteria in patients with cGVHD (N = 42). The 5 patients who had no response assessment during the study are included in the denominator in this intent-to-treat analysis. Reasons for discontinuing the study before a response assessment included toxicity (n = 4) and noncompliance with study drug (n = 1).PD, progressive disease; SD, stable disease.
Best cGVHD response. The best cGVHD response was measured based on the 2005 NIH response criteria in patients with cGVHD (N = 42). The 5 patients who had no response assessment during the study are included in the denominator in this intent-to-treat analysis. Reasons for discontinuing the study before a response assessment included toxicity (n = 4) and noncompliance with study drug (n = 1).PD, progressive disease; SD, stable disease.
Analysis by organ domain showed similar rates of response in the skin (88%), mouth (88%), and gastrointestinal organs (91%). Of 25 responders with ≥2 involved organs, 20 (80%) showed a response in ≥2 organs (Table 3). Ten of 11 patients who were previously treated with rituximab had response assessments; 7 (64%) of these patients responded to ibrutinib. Patients with steroid-dependent cGVHD appeared to have somewhat better responses to ibrutinib than patients with steroid-refractory or both steroid-dependent and -refractory cGVHD with best ORRs of 75% vs 50% vs 50% and CR rates of 25% vs 17% vs 13%, respectively. There did not appear to be a substantial difference in best response between patients using additional immunosuppressants at baseline (n = 22) when compared with those who did not (n = 20) with ORRs of 64% vs 70% and CR rates of 18% vs 25%.
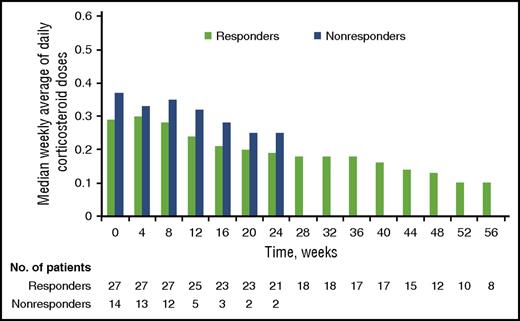
The median corticosteroid dose among responders decreased from 0.29 mg/kg per day (range, 0.06-1.30 mg/kg per day) at baseline (n = 42) to 0.12 mg/kg per day (range, 0.00-0.18 mg/kg per day) at week 49 (n = 12) (Figure 2). Five responders completely discontinued corticosteroids during response to ibrutinib treatment. Overall, 26 patients (62%) reached a corticosteroid dose of <0.15 mg/kg per day during the study.
Change in corticosteroid doses over time. Median change in weekly average of daily corticosteroid doses for responders over time. Responders include patients with a best overall response of CR and PR (n = 28). Nonresponders include patients with stable disease, patients with progressive disease, and patients who were not evaluable for response (n = 14).
Change in corticosteroid doses over time. Median change in weekly average of daily corticosteroid doses for responders over time. Responders include patients with a best overall response of CR and PR (n = 28). Nonresponders include patients with stable disease, patients with progressive disease, and patients who were not evaluable for response (n = 14).
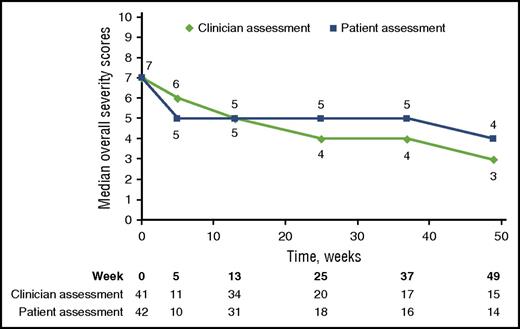
The ORR results were supported by exploratory analyses of patient-reported symptoms, which showed clinically meaningful improvements (at least a 7-point decrease in Lee cGVHD Symptom Scale overall summary score) in 10 of the 42 (24%) treated patients on at least 2 consecutive visits. Clinically meaningful improvement in summary scores was reported for 17 of 28 (61%) responders and 1 of 14 (7%) nonresponders. The median Total Summary Score for responders decreased from 32.8 (n = 28) to 25.7 at week 49 (n = 15). Median overall clinician-assessed cGVHD severity score improved from 7 (n = 41) to 3 at week 49 (n = 15). A corresponding improvement in median patient-reported overall cGVHD score from 7 (n = 42) to 4 at week 49 (n = 14) was reported in the all-treated population (Figure 3).
Improvement in cGVHD symptoms and severity. Change in clinician-assessed and patient-reported severity of cGVHD over time.
Improvement in cGVHD symptoms and severity. Change in clinician-assessed and patient-reported severity of cGVHD over time.
Pharmacodynamic and biomarker studies
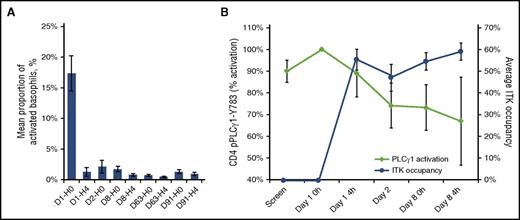
Pharmacodynamic studies showed that mean steady-state occupancy levels of BTK and ITK were 93% (range, 46% to 99%; n = 36) and 37% (range, 0% to 71%; n = 38), respectively, on day 8 of treatment. These steady-state occupancy levels were observed as early as 4 hours after treatment initiation and persisted for the analysis period. BTK occupancy was sufficient to effectively block 91% of BTK-driven basophil activation in an ex vivo IgE stimulation assay. Furthermore, measurement of ITK-mediated activation of PLCγ1-Y783 in CD4 T cells conducted for 4 patients revealed that ITK kinase function was inhibited by a mean of 73% (range, 52% to 86%) on day 8 (Figure 4).
Pharmacodynamic analyses of BTK and ITK from peripheral blood samples. (A) IgE-induced basophil activation. Activated basophils (CD123+, HLA-DR−, CD63+) from 32 patients are shown as a percentage of total basophils (CD123+, HLA-DR−). (B) PLCγ1 activation in CD4 T cells as measured by phosphorylation of Y783 (in red), as well as ITK occupancy (in blue) from 4 patients. Treatment day and hour post–ibrutinib administration are denoted as day number (D) − hour (0 or 4); 0 hour denotes a predose sample.
Pharmacodynamic analyses of BTK and ITK from peripheral blood samples. (A) IgE-induced basophil activation. Activated basophils (CD123+, HLA-DR−, CD63+) from 32 patients are shown as a percentage of total basophils (CD123+, HLA-DR−). (B) PLCγ1 activation in CD4 T cells as measured by phosphorylation of Y783 (in red), as well as ITK occupancy (in blue) from 4 patients. Treatment day and hour post–ibrutinib administration are denoted as day number (D) − hour (0 or 4); 0 hour denotes a predose sample.
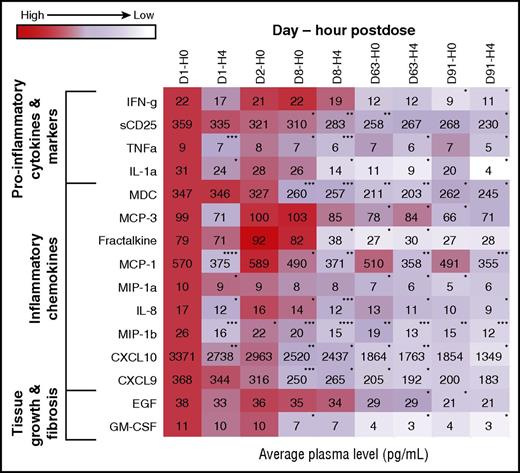
A biomarker analysis based on samples from all 42 patients showed a significant reduction in soluble plasma factors that are markers of inflammation and lymphocyte activation, including tumor necrosis factor-α and soluble CD25. Reductions in several chemotactic factors, including C-X-C motif chemokine ligand 9 (CXCL9) and C-X-C motif chemokine ligand 10 (CXCL10), were observed. An analysis of factors associated with tissue fibrosis revealed reductions in epidermal growth factor and granulocyte-macrophage colony-stimulating factor. These changes occurred after ibrutinib was administered, and an overall downward trend was maintained for measured time points (Figure 5).
Heat map of change in biomarker levels from baseline by time after ibrutinib dose. Heat map of chemokines, cytokines, or factors associated with fibrosis that showed a significant change in levels for at least 1 time point posttreatment. The values at each time point are expressed as a proportion of the baseline value and depicted as a heat map. *P < .05; **P < .01; ***P < .001; ****P < .0001. CXCL9, C-X-C motif chemokine ligand 9; CXCL10, C-X-C motif chemokine ligand 10; EGF, epidermal growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-g, interferon-γ; IL-1a, interleukin-1α; IL-8, interleukin-8; MCP-1, monocyte chemotactic proteins 1; MCP-3, macrophage-derived chemokine, monocyte chemotactic proteins 3; MDC, macrophage-derived chemokine; MIP-1a, macrophage-inflammatory proteins 1α; MIP-1b, macrophage-inflammatory proteins 1β; sCD25, soluble CD25; TNFa, tumor necrosis factor-α.
Heat map of change in biomarker levels from baseline by time after ibrutinib dose. Heat map of chemokines, cytokines, or factors associated with fibrosis that showed a significant change in levels for at least 1 time point posttreatment. The values at each time point are expressed as a proportion of the baseline value and depicted as a heat map. *P < .05; **P < .01; ***P < .001; ****P < .0001. CXCL9, C-X-C motif chemokine ligand 9; CXCL10, C-X-C motif chemokine ligand 10; EGF, epidermal growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-g, interferon-γ; IL-1a, interleukin-1α; IL-8, interleukin-8; MCP-1, monocyte chemotactic proteins 1; MCP-3, macrophage-derived chemokine, monocyte chemotactic proteins 3; MDC, macrophage-derived chemokine; MIP-1a, macrophage-inflammatory proteins 1α; MIP-1b, macrophage-inflammatory proteins 1β; sCD25, soluble CD25; TNFa, tumor necrosis factor-α.
Discussion
Treatment with ibrutinib in patients with cGVHD that had failed 1 or more lines of systemic therapy resulted in a high frequency of sustained responses. The study population was heterogeneous, representative of many cGVHD patients requiring additional systemic therapy. Using the NIH cGVHD Consensus panel response criteria, ibrutinib treatment yielded an ORR for cGVHD of 67% in these pretreated patients, with nearly one-third achieving a CR. The ORR in the response-evaluable patient population was 76%, allowing for comparison with historical reports of efficacy of other second-line therapies. Sustained response was observed with 71% of responders maintaining response for ≥20 weeks. Similar response rates were seen across affected organs, and 80% of patients with multiple organ involvement showed response in ≥2 organs. Observed responses were associated with decreased corticosteroid use and an improvement in cGVHD symptoms.
Although response rates ranging from 20% to 70% have been reported in studies of second-line agents for cGVHD,15 these results were often based on small, uncontrolled trials with suboptimal study designs. Subsequent randomized studies to confirm the initial results were all unsuccessful. To explain the discrepancy between the results of early trials and subsequent randomized studies, Martin et al analyzed 60 early cGHVD trials using 10 clinical trial quality indicators.30 The analysis of these trials, most of which were conducted before publication of the NIH standardized response criteria for cGVHD,27 showed that the studies satisfied an average of only 2.5 of the 10 clinical trial quality measures.30 The investigators concluded that poor study design, including lack of rigorous entry, organ response, and overall response criteria, may have biased the reported efficacy in the early phase studies, leading to later unsuccessful controlled studies with the same agents. In 2005, the NIH cGVHD Consensus panel developed response criteria to improve evaluation of cGVHD response27 ; implementation of these criteria has been shown to reduce bias in the reported efficacy of second-line treatment of cGVHD.31 Although the NIH criteria were created to provide the most objective assessments of response, they still represent a subjective determination of cGVHD activity by the clinician. Because the present study used the NIH-defined response criteria, the ORR in this study is more robust than those reported in historical studies.
This cGVHD study is the first to report sustained response as an efficacy endpoint. This end point is clinically relevant because cGVHD patients generally require therapy for an extended period, and short-term responses do not allow for resolution of disabling symptoms or tapering of corticosteroids. Without a sustained response, the most common approach to improve response is the addition of new agents to ongoing therapy with corticosteroids.32 Nearly three-quarters of responders in this study maintained their response for ≥20 weeks, and this was accompanied by meaningful reductions or discontinuation in corticosteroid use. Although the reduction of steroid doses in this open-label study could have been influenced by the investigators’ assessment of response, our results suggest that ibrutinib may have a steroid-sparing effect, which could reduce the morbidity associated with long-term corticosteroid use.33
The clinical efficacy of ibrutinib in cGVHD is further supported by an overall improvement in Lee cGVHD Symptom Scale score in 61% of responders. The Lee Symptom Scale directly measures the effect of ibrutinib on patient quality of life and symptom burden based on the multiorgan manifestations of the disease.4 The positive effect on symptom burden among responders was reinforced by a decrease in cGVHD severity scores reported by both clinicians and patients.
Ibrutinib showed an acceptable safety profile in this pretreated cGVHD patient population, with AEs similar to those observed in ibrutinib-treated patients with B-cell malignancies and for patients with cGVHD treated with concomitant corticosteroids. One-third of patients discontinued treatment because of AEs. The relatively higher discontinuation rate for AEs compared with those observed in patients with B-cell malignancies may reflect that most patients had a low Karnofsky Performance Status score, comorbidities, and reduced fitness level, consistent with the relapsed cGVHD population on ongoing immunosuppressants. As expected for cGVHD patients on long-term corticosteroid treatment, AEs, including hyperglycemia and infections, were observed. AEs associated with ibrutinib, such as major bleeding and atrial fibrillation, occurred infrequently in this population.
BTK occupancy of >90% was observed, and target occupancy results showed higher average engagement of BTK than ITK. Functionally relevant blockade of both kinases was observed, indicating that both BTK and ITK were sufficiently inhibited to induce biologic impact. These data are consistent with those of prior in vivo CLL patient experiences.24
Ibrutinib is unique in its ability to exert effects on B cells and T cells, both of which have been implicated in the pathogenesis of cGVHD.25 Biomarker analyses conducted on this population support the notion that ibrutinib targets the cellular and molecular pathways responsible for cGVHD. A striking number of cGVHD-related inflammatory, chemotactic, and fibrotic factors were significantly decreased across all patients following ibrutinib therapy, suggesting that ibrutinib could be impacting resolution of allogeneic inflammation at the cellular level. Decreases in markers of inflammation and lymphocyte activation have been reported to correlate with cGVHD development and severity.34,35 Reduction in plasma concentrations of chemotactic factors indicates a reduction in immune cell recruitment to sites of active cGVHD.36-39 Furthermore, changes in levels of tissue factors associated with fibrosis suggest a decreased propensity for tissue fibrosis and restoration of normal organ function.40,41 These findings, together with the demonstration of clinical response across multiple organs, support the hypothesis that ibrutinib is affecting cGVHD at a pathogenic level and not just treating symptoms of cGVHD.
In the absence of an approved treatment of cGVHD that does not respond adequately to corticosteroids, there is currently no consensus on the optimal second-line treatment. Treatment choices are empirical and based on factors such as physician experience, ease of use, need for monitoring, risk of toxicity, and potential exacerbation of a preexisting comorbidity.15 The objective and sustained response observed with ibrutinib, the ability to decrease or discontinue corticosteroid doses, and the improvement in symptoms among responders provide evidence that cGVHD patients derived broad benefit from ibrutinib treatment. The clinical benefit and acceptable safety profile, coupled with ibrutinib’s ease of administration in an outpatient setting with once-daily oral dosing, make ibrutinib a promising treatment option for patients with cGVHD whose disease failed to respond to frontline therapy. Further validation is necessary, and a randomized phase 3 trial in the frontline setting (NCT02959944) is underway.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the investigators and coordinators at each of the clinical sites, the patients who participated in this trial and their families, and the employees of Pharmacyclics who contributed to the design and implementation of the trial. The authors also thank John S. Hill, Melissa A. Hopper, Fabiola M. Bittencourt, Pak-Yan Patricia Cheung, and Bita Sahaf for biomarker assays and pharmacodynamic analysis.
This study was supported by Pharmacyclics LLC, an AbbVie Company. Supriya Srinivasan, funded by Pharmacyclics LLC, an AbbVie Company, provided medical writing support in the preparation of the manuscript.
Authorship
Contribution: D.M., B.R.B., L.S., and D.F.J. designed the study; D.M, C.S.C., M.A., E.K.W., M.J., M.E.F., A.C.L., R.N., I.P., and S.J. enrolled patients and collected data; Y.L. and S.C. performed statistical analysis of data; L.S., I.L., Y.L., and S.C. assembled the data; Y.L., S.C., L.S., and D.F.J. analyzed and interpreted the data; J.D. conducted the biomarker analyses; J.D. and B.R.B. reviewed and analyzed the biomarker data; D.M. and L.S. wrote the first draft of the manuscript; and all authors were involved in the preparation of and revisions to the manuscript and approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: D.M. served in a consultancy/advisory role for Pharmacyclics LLC, an AbbVie Company, Sanofi, Adaptive Biotechnologies, Kite, Genentech, and Velos; received research funding from Pharmacyclics LLC, an AbbVie Company; travel accommodations from Pharmacyclics LLC, an AbbVie Company, and Sanofi Oncology; and patents, royalties, and other intellectual property from Pharmacyclics LLC, an AbbVie Company. C.S.C. served in a consulting or advisory role with Pfizer, Kite, Bristol-Myers Squibb, Incyte, Astellas, and Pharmacyclics, an AbbVie Company. M.A. served in a consultancy/advisory role for Takeda Oncology. E.K.W. shared equity ownership with Cambium; received honoraria from Novartis; patents/royalties/other intellectual property and other relationship with Cambium Medical Technologies; received travel expenses with Novartis; served in a consulting/advisory role with Novartis, Alenon, Seattle Genetics, Mesoblast, and Aiye; and received research funding from Novartis and Sanofi. M.J. served in a consultancy/advisory role and received research funding from Theracos and Janssen. M.E.F. received research funding from Pharmacyclics LLC, an AbbVie Company. A.C.L. served in a consulting/advisory role with Amgen, Jazz, and Pharmacyclics LLC, an AbbVie Company, and received research funding from Astellas, Novartis, and Pharmacyclics LLC, an AbbVie Company. R.N. served in a consultancy/advisory role for Amgen and Seattle Genetics. B.R.B. served in a consultancy/advisory role with Tobira Therapeutics, Vulcan Capital, Idera Pharma, Sidley Austin LLP, Merck Sharpe & Dohme Corp, Merck Serono, Fate Therapeutics, Bristol-Myers Squibb, Sidley Austin, Kadmon Pharmaceuticals Inc, Kymab Scientific, Five Prime Therapeutics, Vitae Pharmaceuticals Inc, and Flx Bio; received research funding from Kadmon Corporation; and received patents/royalties/other intellectual property as an individual (no company). Y.L. was employed with Pharmacyclics LLC, an AbbVie Company, and held equity ownership with AbbVie. S.C. was employed with Pharmacyclics LLC, an AbbVie Company, and held equity ownership with AbbVie, Johnson & Johnson, and Portola. I.L. was employed with The Permanente Medical Group, Gilead Sciences, and Pharmacyclics LLC, an AbbVie Company, and held equity ownership with The Permanente Medical Group, Gilead Sciences, Reviva Pharmaceuticals, Clovis, Infinity, and AbbVie. J.D. was employed with Pharmacyclics LLC, an AbbVie Company, and held equity ownership with AbbVie. D.F.J. was employed with Pharmacyclics LLC, an AbbVie Company, and held equity ownership with AbbVie. L.S. was employed with Pharmacyclics LLC, an AbbVie Company, and held equity ownership with AbbVie. S.J. served in a consultancy/advisory role with Pharmacyclics LLC, an AbbVie Company, and received research funding from Pharmacyclics LLC, an AbbVie Company. I.P. declares no competing financial interests.
Correspondence: David Miklos, Stanford Cancer Institute, Stanford University, 875 Blake Wilbur Dr, Stanford, CA 94305; e-mail: dmiklos@stanford.edu.