In this issue of Blood, Mantuano et al in the Gonias laboratory elucidate a novel, protease-independent signaling pathway by which tissue plasminogen activator (tPA) negatively regulates the innate immune response of macrophages and neutralizes lipopolysaccharide (LPS) toxicity in vivo.1
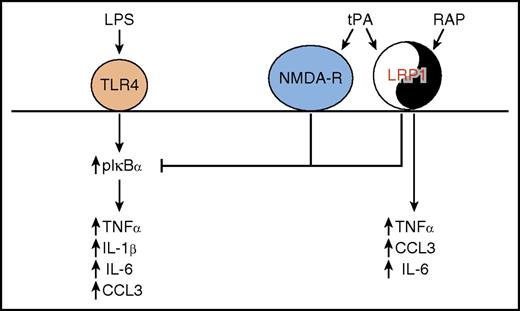
tPA regulates proinflammatory macrophage activation. In a yin-yang manner, LRP1 mediates both proinflammatory and anti-inflammatory effects on macrophage cytokine expression, through ligand-specific cell signaling. In an anti-inflammatory mechanism, tPA interacts with both NMDA-R and LRP1 to block the sustained IκBα phosphorylation induced when LPS interacts with Toll-like receptor 4 (TLR4). This results in blockade of the LPS-induced expression of TNFα, IL-1β, IL-6, and CCL3. In a proinflammatory mechanism, RAP interacts with LRP1 to induce expression of TNFα, IL-6, and CCL3.
tPA regulates proinflammatory macrophage activation. In a yin-yang manner, LRP1 mediates both proinflammatory and anti-inflammatory effects on macrophage cytokine expression, through ligand-specific cell signaling. In an anti-inflammatory mechanism, tPA interacts with both NMDA-R and LRP1 to block the sustained IκBα phosphorylation induced when LPS interacts with Toll-like receptor 4 (TLR4). This results in blockade of the LPS-induced expression of TNFα, IL-1β, IL-6, and CCL3. In a proinflammatory mechanism, RAP interacts with LRP1 to induce expression of TNFα, IL-6, and CCL3.
tPA is 1 of 2 major physiological activators of plasminogen, the circulating zymogen of the enzyme, plasmin, the predominant enzyme responsible for degradation of fibrin clots. Beyond fibrinolysis, a role for the plasminogen activation system in the proinflammatory phase of the immune response is well documented. Cell surface plasmin is required for macrophage recruitment in response to inflammatory stimuli (reviewed in Miles et al2 ). This is accomplished both by plasmic cleavage of extracellular matrix components3 and by plasmin-dependent induction of cytokines and intracellular signaling events to potentiate the early inflammatory response.4,5 More recently, a role for the plasminogen activation system in the anti-inflammatory response has emerged. The anti-inflammatory response is critical because unchecked inflammation can result in chronic and autoimmune disease.6 Notably, recent studies have established that plasminogen exerts a profound effect on macrophage phagocytosis,7 efferocytosis,8 macrophage reprogramming,8 and clearance of extravascular fibrin,9 key steps in the resolution of inflammation. Here, Mantuano et al have identified a novel signaling pathway by which tPA attenuates the proinflammatory response via a unique mechanism that is likely to be independent of plasminogen.
The authors show that the proinflammatory response of macrophages to LPS is disrupted in the presence of tPA. LPS treatment induced IκBα phosphorylation, while decreasing total IκBα and activating extracellular signal-regulated kinase 1/2 (ERK1/2), as previously documented.10 These key events were blocked in the presence of tPA. Time course experiments demonstrated that tPA does not prevent LPS-dependent IκBα phosphorylation initially, but functions to reverse it. Most importantly, from the standpoint of resolution of the immune response, tPA blocked the LPS-dependent increase in expression of cytokines, tumor necrosis factor α (TNFα), interleukin-1β (IL-1β), IL-6, and CCL3.
The nature of the cellular receptors mediating the tPA anti-inflammatory effect was complex. Blockade of LPS-induced signaling and cytokine responses by tPA required low-density lipoprotein receptor-related protein 1 (LRP1). However, other ligands that also bind to LRP1, including receptor-associated protein (RAP) and lactoferrin, had the opposite effect, stimulating cytokine synthesis. Treatment of macrophages with RAP activated the major MAPKs ERK1/2, JNK, and p38 MAPK, whereas tPA activated only ERK1/2. The authors concluded that ERK1/2 activation is a common property of both proinflammatory and anti-inflammatory LRP1 ligands. For the first time, the N-methyl-d-aspartic acid receptor (NMDA-R) was identified as playing a major role in the tPA response on macrophages. NMDA-R was not involved in the response to RAP. Thus, the difference in activity of tPA and RAP may reflect the requirement for NMDA-R to generate an anti-inflammatory response (see figure). Notably, both enzymatically active and inactive tPA elicited the same effects on macrophage inflammatory signaling. Thus, the plasminogen-activating ability of tPA does not appear to play a role. In addition to the protease domain, the tPA protein includes a fibronectin type I domain, an epidermal growth factor–like domain, and 2 kringle domains. It is possible that one of these domains may interact with LRP1 while another interacts with NMDA-R in order to effect tPA-dependent signaling.
Finally, exciting results showed that tPA blocked LPS toxicity in vivo. When mice were treated with tPA following LPS injection, all of the mice survived over the time course of the experiment, whereas 83% of mice treated with LPS died within 72 hours. The results of Mantuano et al provide a strong rationale for future studies to investigate tPA as a proresolving mediator of inflammation; to address the clinical efficacy of enzymatically inactive tPA in sepsis, autoimmune disorders, and other inflammatory states; and also to test the ability of specific domains of tPA to recapitulate the response of intact tPA.
Conflict-of-interest disclosure: The authors declare no competing financial interests.