Key Points
Genetic analysis reveals frequent 9p24.1/PD-L1/PD-L2 copy-number alterations and increased expression of the PD-1 ligands in PCNSL and PTL.
PD-1 blockade with nivolumab demonstrated activity in patients with relapsed/refractory PCNSL and PTL.
Abstract
Primary central nervous system (CNS) lymphoma (PCNSL) and primary testicular lymphoma (PTL) are rare extranodal large B-cell lymphomas with similar genetic signatures. There are no standard-of-care treatment options for patients with relapsed and refractory PCNSL and PTL, and the overall prognosis is poor. PCNSLs and PTLs exhibit frequent 9p24.1 copy-number alterations and infrequent translocations of 9p24.1 and associated increased expression of the programmed cell death protein 1 (PD-1) ligands, PD-L1 and PD-L2. The activity of PD-1 blockade in other lymphomas with 9p24.1 alterations prompted us to test the efficacy of the anti–PD1 antibody, nivolumab, in 4 patients with relapsed/refractory PCNSL and 1 patient with CNS relapse of PTL. All 5 patients had clinical and radiographic responses to PD-1 blockade, and 3 patients remain progression-free at 13+ to 17+ months. Our data suggest that nivolumab is active in relapsed/refractory PCNSL and PTL and support further investigation of PD-1 blockade in these diseases.
Introduction
Primary central nervous system (CNS) lymphoma (PCNSL) and primary testicular lymphoma (PTL) are rare and aggressive extranodal non-Hodgkin lymphomas with shared molecular features and a poor prognosis.1-5 PCNSLs arise from the brain, spinal cord, leptomeninges, or eye in the absence of prior or concurrent systemic disease. The median overall survival of patients with PCNSL is 30 to 50 months, with recurrence rates of almost 50% within the first 2 years of diagnosis.1,3 Additionally, one-third of patients with PCNSL are refractory to initial treatment.2,3 Treatment options for relapsed/refractory PCNSL include whole-brain radiotherapy (WBRT) or high-dose chemotherapy and autologous stem cell transplantation in younger patients who did not receive these as a part of initial treatment, or conventional chemotherapy. Responses to these regimens are poor and often not durable.6-8 Moreover, WBRT and high-dose chemotherapy and autologous stem cell transplantation can lead to considerable treatment-related neurotoxicity and morbidity, particularly when used for salvage, and may not be ideal for older patients.9-11 In a disease where the median age at diagnosis is 65 years, these treatments are generally not good options. PTLs typically occur in elderly men and frequently relapse in extranodal sites, including the CNS.5,12 Treatment options are also limited for patients with CNS recurrences of PTL.
In recent comprehensive genomic analyses, PCNSL and PTL were found to have shared molecular features.4,13-16 Our recent genetic evaluation also revealed frequent 9p24.1/PD-L1(CD274)/PD-L2 (PDCD1LG2) copy-number alterations and associated increased expression of the programmed cell death protein 1 (PD-1) ligands PD-L1 and PD-L2 in PCNSL and PTL.4 Less commonly, chromosomal rearrangements involving PD-L1 or PD-L2 and selective overexpression of the respective ligand were identified.4
PD-L1 and PD-L2 engage the PD-1 receptor on T cells and activate PD-1 signaling. This leads to a reversible state of T-cell “exhaustion” characterized by decreased T-cell receptor activation, reduced T-cell proliferation and survival, perturbed metabolism, and altered transcription factor expression.17 The activity of PD-1 blockade in other lymphomas with 9p24.1 alterations such as classical Hodgkin lymphoma18-22 prompted us to evaluate this approach in relapsed/refractory PCNSL and PTL.
Study design
Here, we describe 4 patients with recurrent/refractory PCNSL and 1 patient with CNS recurrence of PTL who were treated with nivolumab, a human immunoglobulin G4 monoclonal antibody that targets PD-1 and blocks engagement of the PD-1 ligands. Informed consent for off-label use of nivolumab was obtained from all 5 patients. This was a consecutive series of 5 patients, from 3 institutions (Dana-Farber Cancer Institute/Brigham and Women’s Hospital and New York Presbyterian Hospital), who began nivolumab therapy between August and December 2015 and had a minimum of 13 months follow-up.
Results and discussion
Patients and therapy
The median age at the time of recurrent/refractory disease was 64 years (range, 54-85 years), and the median Karnofsky performance status (KPS) was 70% (range, 40% to 80%). Four patients had multiply recurrent PCNSL (3 patients) or CNS relapse of PTL (1 patient), and 1 patient had primary refractory PCNSL (Table 1). All patients had brain parenchymal disease; 1 patient also had leptomeningeal and intraocular involvement.
All patients had been treated with standard-of-care regimens and had no other available options. Patients with PCNSL had received prior treatments, including high-dose methotrexate–based chemotherapy, pemetrexed, high-dose cytarabine, and WBRT. The patient with CNS recurrence of PTL was previously treated with high-dose methotrexate followed by thiotepa-based conditioning chemotherapy and subsequent autologous stem cell transplantation. All 5 patients received nivolumab at 3 mg/kg IV every 2 weeks. In 1 patient with rituximab-refractory PCNSL, rituximab was continued for 3 doses after beginning nivolumab treatment. Two other patients received whole brain or focal radiation immediately prior to the initiation of nivolumab. Only 1 of the 5 patients was on corticosteroids (dexamethasone at 2mg oral daily) at the time of starting treatment with nivolumab. In this patient, corticosteroids were tapered and discontinued within a month.
Toxicities
The toxicities of nivolumab therapy included grade 2 pruritus in 1 patient and grade 2 fatigue in another patient, both thought to be drug related. One patient developed worsening of a baseline renal insufficiency (grade 4) prompting discontinuation of nivolumab after 3 doses and initiation of hemodialysis (Table 1, patient 2). Corticosteroids did not improve this condition. Renal biopsy was performed and revealed advanced changes indicative of chronic renal failure (tubular atrophy, interstitial fibrosis, and severe arterial sclerosis) with no evidence of the interstitial nephritis seen with immunotherapies.23
Radiographic responses
All 5 patients had objective radiographic responses to treatment with nivolumab including 4 complete responses and 1 partial response (Table 1). Responses were confirmed and none of the patients were on corticosteroids during radiographic assessment of response. The median number of nivolumab treatments to objective radiographic response was 3 (range, 2-4).
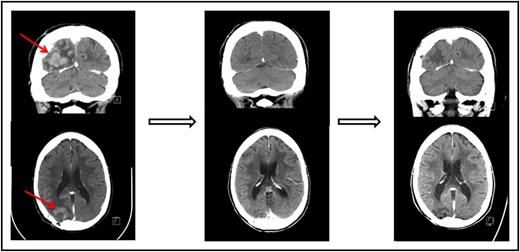
One of the patients who achieved a complete response to nivolumab had primary refractory PCNSL (Figure 1 and Table 1, patient 1). One patient who achieved only a partial response to prior WBRT had a further decrease in enhancing disease on nivolumab therapy (Table 1, patient 3). An additional patient who achieved only a partial response to prior focal radiation obtained a complete radiographic response to nivolumab with resolution of parenchymal and leptomeningeal involvement (Table 1, patient 5). This patient had persistent intraocular disease that was confirmed by vitrectomy and treated with ocular radiation (Table 1, patient 5).
Pre- and posttreatment head CTs with contrast in a patient with primary refractory PCNSL. (Left) The contrast-enhancing lesion before treatment with nivolumab. (Middle) Complete response following 2 months therapy with nivolumab. (Right) Continued complete response 13 months following initiation of therapy.
Pre- and posttreatment head CTs with contrast in a patient with primary refractory PCNSL. (Left) The contrast-enhancing lesion before treatment with nivolumab. (Middle) Complete response following 2 months therapy with nivolumab. (Right) Continued complete response 13 months following initiation of therapy.
Symptomatic responses and progression-free survival
Four patients who were symptomatic at the initiation of nivolumab therapy had complete or near-complete resolution of neurologic signs/symptoms and an improvement in KPS (Table 1). All patients are alive at a median follow up of 17 months. One patient with PCNSL developed a systemic recurrence without detectable CNS involvement at 14 months (Table 1, patient 4). The patient who received only 3 doses of nivolumab developed recurrent CNS disease at 17 months (Table 1, patient 2). Three patients remain radiographically and clinically progression-free at 13+ to 17+ months following the initiation of nivolumab therapy (Table 1).
Summary
Recurrent/refractory PCNSL and PTL remains a major unmet need in oncology today. In this case series, 4 patients had multiple recurrences and 1 patient had primary refractory disease following standard therapies. All 5 patients had clinical and radiographic responses to off-label treatment with nivolumab and 3 patients remain progression-free at 13+ to 17+ months.
The observed clinical responses in this small case series and the recently reported 9p24.1 alterations in PCNSL and PTL4 support the prospective evaluation of PD-1 blockade in these diseases. For these reasons, a multi-institutional phase 2 trial of nivolumab in recurrent and refractory PCNSL and PTL patients (CA209-647, #NCT02857426) is now open to accrual.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: All authors contributed to data collection and interpretation and writing of the manuscript.
Conflict-of-interest disclosure: F.M.I. is a consultant for Merck, Novocure, Regeneron, Abbvie, and Alexion and received research support from Novocure, Bristol-Myers Squibb, Celldex, Northwest Biotherapeutics, Stemline, Regeneron, Incyte, Immunocellular Therapeutics, and Merck. A.L. is a member of the Bristol-Myers Squibb Data & Safety Monitoring Board. P.A. is a consultant for Merck, Bristol-Myers Squibb, and Infinity and received research support from Bristol-Myers Squibb, Merck, Affimed, Pfizer, Otsuka, Sequenta, and Sigma Tau. S.J.R. received research funding from Bristol-Myers Squibb. M.A.S. is a consultant for Bristol-Myers Squibb, Cell Signaling, Gilead, Takeda, Seattle Genetics, and AstraZeneca and received research funding from Bristol-Myers Squibb and Bayer. The remaining authors declare no competing financial interests.
Correspondence: Lakshmi Nayak, Dana-Farber Cancer Institute, 450 Brookline Ave, DA 2120, Boston, MA 02215; e-mail: lakshmi_nayak@dfci.harvard.edu.