To the editor:
In 2012, the American Society of Clinical Oncology (ASCO) published guidelines regarding the dosing of chemotherapy for obese patients with cancer.1 In these patients, empiric dose reductions are often performed, usually by capping body surface area (BSA) at 2 m2 because of concerns regarding toxicity. However, there is no evidence that administering a full dose of chemotherapy in obese patients with solid cancer is associated with an increased toxicity.1 In addition, the ASCO guidelines highlight the negative impact on prognosis of reduced doses of chemotherapy in obese patients with solid tumors and thus recommend the use of full weight–based doses of cytotoxic chemotherapy, particularly in curable cancers.1 However, the impact of chemotherapy dosing has not been evaluated in non-Hodgkin lymphoma (NHL) patients with elevated BSA.
In this study, we aimed to evaluate the impact of doxorubicin dose capping in patients with diffuse large B-cell lymphoma (DLBCL). DLBCL is a curable disease in which relative dose intensity has been described to be associated with treatment efficacy.2 However, clinicians may be concerned with the toxicity of high doses of anthracyclines in patients with elevated BSA.
To address this question, we analyzed all DLBCL patients treated in first line in 1 of the following prospective Lymphoma Study Association, Group of the Adult Lymphoma, and/or Acute Leukaemia and Blood Diseases West-East Group trials: LNH98-5, LNH-75, LNH03-2B, and LNH03-6B3-6 (supplemental Figure 1, available on the Blood Web site). All patients received rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or R-CHOP-like (mostly rituximab, doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone [R-ACVBP]) regimens. A theoretical BSA was calculated with Mosteller’s formula for each patient according to their weight and height at inclusion. According to the dose planned in the protocol (theoretical dose) and the dose that was actually administered (observed dose), we could determine if the dose had been capped or not. Cutoff value for BSA was 2.1 m2. Patients with a BSA of >2.1 m2 and doxorubicin dose capped at a BSA of 2 m2 received <95% of the theoretical dose. Our cohort was divided into 3 groups: BSA < 2.1 m2 (theoretical BSA and observed BSA < 2.1 m2), capped BSA ≥ 2.1 m2 (theoretical BSA ≥ 2.1 m2 and observed BSA < 2.1 m2), and uncapped BSA ≥ 2.1 m2 (theoretical BSA and observed BSA ≥ 2.1 m2). Flowchart for patient selection is presented in supplemental Figure 1.
A total of 1384 patients were included in the analysis: 89% (N = 1232) of the patients had a BSA < 2.1 m2 (BSA < 2.1 m2 group), 8.6% (N = 119) had a BSA ≥ 2.1 m2 and received a capped dose of doxorubicin (capped BSA ≥ 2.1 m2 group), and 2.4% (N = 33) had a BSA ≥ 2.1 m2 and received a full weight dose of doxorubicin (uncapped BSA ≥ 2.1 m2 group). Compared with patients with a BSA ≥ 2.1 m2, the patients with a BSA < 2.1 m2 were older (74% of the patients were >60 years old vs 55.5% of patients in the capped BSA ≥ 2.1 m2 group and 51.5% of patients in the uncapped BSA ≥ 2.1 m2 group, P < .001), had a lower body mass index (BMI; 58.8% had a BMI < 25 kg/m2 vs 3.4% and 9.1%, respectively, P < .001), were predominantly women (49.3% vs 10.1% and 6.1%, P < .001), had a higher age-adjusted International Prognostic Index (aaIPI) score (47% had an aaIPI score >1 vs 37.8% and 30.3%, P < .001), and had more B symptoms (36.2% vs 20.2% and 18.2%, P < .001) (supplemental Table 1). Groups were comparable for the following factors: Ann Arbor stage, number of extra nodal lesions, and treatment regimens (R-CHOP, CHOP, and R-ACVBP).
The rate of treatment-related death was 3.9% in the BSA < 2.1 m2 group, 6.7% in the capped BSA ≥ 2.1 m2 group, and 6.1% in the uncapped BSA ≥ 2.1 m2 group. These differences were not statistically significant (P = .293; Table 1).
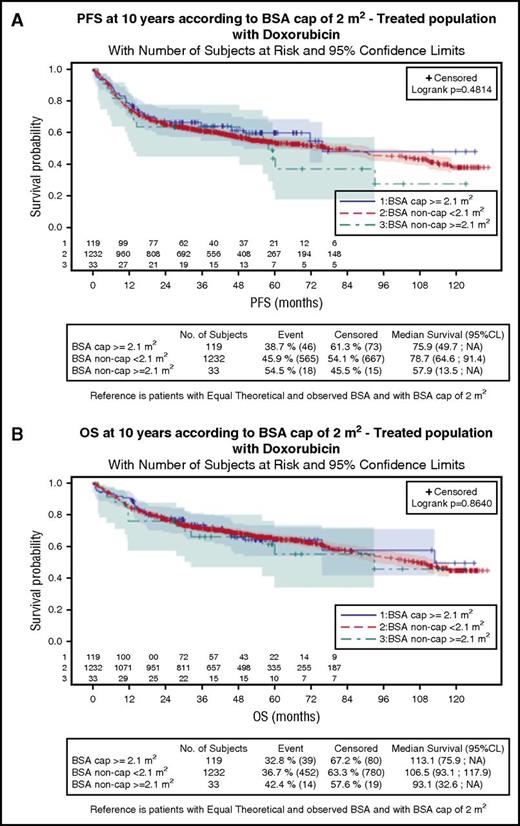
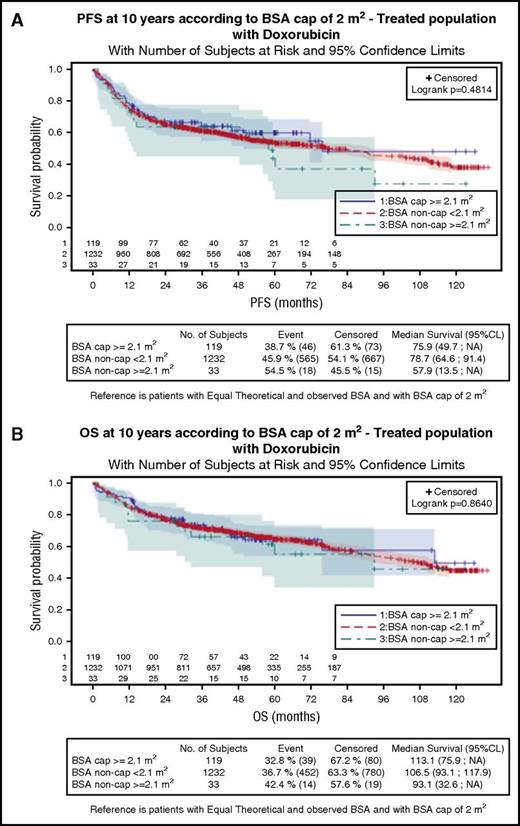
Progression-free survival (PFS) and overall survival (OS) for the 3 groups are shown in Figure 1. Median PFS was 78.7, 75.9, and 57.9 months for patients with BSA < 2.1 m2, capped BSA ≥ 2.1 m2, and uncapped BSA ≥ 2.1 m2, respectively. Median OS was 106.5, 113.1, and 93.1 months, respectively. There was no statistical difference between the groups for PFS or OS (P = .481 and 0.864, respectively) (Table 1). These results remained unchanged after adjusting for sex, aaIPI score, and BMI (supplemental Table 2).
Survival according to BSA and doxorubicin dose adaptation in patients with DLBCL. PFS (A) and OS (B) according to BSA and doxorubicin dose adaptation in patients with DLBCL (Log-rank, P = .481 and P = .864, respectively). CL, confidence limits.
Survival according to BSA and doxorubicin dose adaptation in patients with DLBCL. PFS (A) and OS (B) according to BSA and doxorubicin dose adaptation in patients with DLBCL (Log-rank, P = .481 and P = .864, respectively). CL, confidence limits.
Our study did not show any impact of doxorubicin dose capping at 2 m2 on PFS nor OS in DLBCL patients with elevated BSA. Similar results were reported by Kempf et al in AML patients with an elevated BSA, except for the subgroup of obese patients with a favorable cytogenetic risk.7 Changes in the pharmacokinetics of doxorubicin in patients with elevated BSA may have neutralized the impact of dose capping on the outcome. Indeed, doxorubicin clearance is reduced in obese patients, thereby increasing doxorubicin exposure.8,9 In NHL patients, some retrospective studies have found that the outcome was better10,11 or similar12 in overweight and obese patients receiving full-weight chemotherapy dosing compared with patients with normal BMI. Such difference was not found in the current study.
The treatment-related mortality did not differ between the 3 groups, although high BMI and cumulative dose of anthracycline have been associated with higher risk of anthracycline cardiotoxicity.13 In our study, the influence of confounding factors such as age, sex, performance status, and comorbidities could not be excluded. Ganti et al reported a lower incidence of treatment-related mortality in obese NHL patients, and a trend in overweight patients.14 Other studies reported similar rates of hematologic and nonhematologic toxicities in full-dosed overweight and obese patients compared with normal-weight patients.9,10,12
Interpretation of these results requires caution because of some limitations. First, this is an unplanned retrospective analysis. Second, the sample size of the patients with an elevated BSA and an uncapped dose of doxorubicin is small and may have underpowered the capacity to detect any statistical difference in survival or treatment-related mortality. Indeed, the power estimated for the PFS analysis with this sample size was 60%. Finally, the groups were not comparable for several prognostic factors that may influence prognosis and toxicity.
In conclusion, our study did not demonstrate inferior efficacy when doxorubicin dose was capped at 2 m2 in DLBCL patients with elevated BSA. On the other hand, uncapped dosing of doxorubicin did not seem to increase the incidence of treatment-related mortality. Therefore, these 2 options seem acceptable in DLBCL patients with elevated BSA.
Authorship
Contribution: R.H. and M.-A.L. designed the research, analyzed data and wrote the paper; C.G. and S.B. analyzed data and wrote the paper; R.D., N.M., L.O., B.C., C.H., H.T., G.S., and T.L. provided the data and wrote the paper. All authors reviewed and approved the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roch Houot, Service d'Hématologie Clinique, Hôpital Pontchaillou-CHU de Rennes, 2 rue Henri Le Guilloux, 35033 Rennes Cedex 9, France; e-mail: roch.houot@chu-rennes.fr.
The online version of this article contains a data supplement.
References
Author notes
M.-A.L. and R.H. contributed equally to this study.