To the editor:
Lipocalin-2 is a eukaryotic siderophore-binding protein that prevents the growth and spread of microorganisms that require siderophore-mediated uptake of soluble iron.1 Consequently, lipocalin-2 knockout mice (Lcn2−/− mice) are more susceptible to infection with the siderophore-producing pathogen Klebsiella pneumoniae2 than wild-type (WT) mice. Lipocalin-2 is a major constituent of neutrophil-specific granules3 and can be induced in epithelial cells4-6 and macrophages7 during inflammation. In mice, lipocalin-2 is also produced in the liver as an acute phase protein.8-10
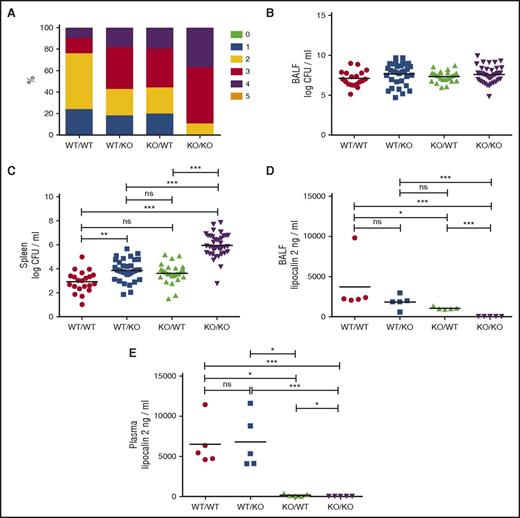
Epithelial cells are important for innate immunity because they provide a physical barrier against invasion of microorganisms, but their role in antimicrobial defense by secreting antibacterial proteins is largely unknown. To this end, we generated mice with chimeric expression of lipocalin-2 by transplanting bone marrow cells to 10- to 12-week-old lethally irradiated female mice. Reconstitution and chimerism was confirmed by flow cytometry (supplemental Figure 1, available on the Blood Web site) and immunohistochemical staining for lipocalin-2 (supplemental Figure 2). Seven weeks posttransplant, reconstituted mice were inoculated with 5 × 106K pneumoniae. Lcn2−/− mice reconstituted with Lcn2−/− bone marrow (henceforth, called KO/KO mice) showed a more severe clinical score phenotype at 24 hours postinoculation compared with WT mice reconstituted with WT bone marrow (WT/WT mice) (Figure 1A). WT mice with Lcn2−/− bone marrow (WT/KO) and Lcn2−/− mice with WT bone marrow (KO/WT) had clinical scores between the KO/KO and WT/WT subgroups.
Lipocalin-2 deficiency impairs clinical status and resistance to dissemination of K pneumoniae, and resistance is not correlated to plasma levels of lipocalin-2. (A) Clinical scores for WT/WT, WT/KO, KO/WT, and KO/KO mice. Clinical score criteria: 0: the mouse is unaffected; 1: the mouse is slightly affected, for instance, slight piloerection or commencing waistline; 2: the mouse is affected (marked waistline, piloerection, or slightly decreased mobility); 3: the mouse is clearly affected (ruffled fur, eyes half closed, marked waistline, hunched posture, affected breathing, or decreased mobility); 4: the mouse is very affected and should be killed (ruffled fur, immobile, cold, eyes closed, or condition will proceed to death within hours); and 5: the mouse is dead. Each of the 6 score levels (0-5) is marked with a color. Clinical score distributions for each of the 4 subgroups are shown in percentages. The percentage of mice scoring a particular score is shown in the color allocated to that score. P = .0009 by χ2. (B) Bacterial counts are expressed as logarithmic transformed (log10) CFUs per milliliter in BALF for each of the 4 subgroups. No statistical significance between groups as tested by analysis of variance with Tukey's multiple comparison test. (C) Bacterial counts in spleen homogenates are expressed as logarithmic transformed (log10) CFU per milliliter. WT/WT: n = 21, WT/KO: n = 33, KO/WT: n = 25, and KO/KO: n = 38. (D) Lipocalin-2 concentrations in BALF from 5 randomly selected mice from each of the 4 transplanted subgroups 24 hours after inoculation analyzed by enzyme-linked immunosorbent assay. (E) Lipocalin-2 concentrations in plasma from the same mice analyzed in (D). Horizontal lines indicate means. *P < .05, **P < .01, and ***P < .001 by analysis of variance with Tukey's multiple comparison test.
Lipocalin-2 deficiency impairs clinical status and resistance to dissemination of K pneumoniae, and resistance is not correlated to plasma levels of lipocalin-2. (A) Clinical scores for WT/WT, WT/KO, KO/WT, and KO/KO mice. Clinical score criteria: 0: the mouse is unaffected; 1: the mouse is slightly affected, for instance, slight piloerection or commencing waistline; 2: the mouse is affected (marked waistline, piloerection, or slightly decreased mobility); 3: the mouse is clearly affected (ruffled fur, eyes half closed, marked waistline, hunched posture, affected breathing, or decreased mobility); 4: the mouse is very affected and should be killed (ruffled fur, immobile, cold, eyes closed, or condition will proceed to death within hours); and 5: the mouse is dead. Each of the 6 score levels (0-5) is marked with a color. Clinical score distributions for each of the 4 subgroups are shown in percentages. The percentage of mice scoring a particular score is shown in the color allocated to that score. P = .0009 by χ2. (B) Bacterial counts are expressed as logarithmic transformed (log10) CFUs per milliliter in BALF for each of the 4 subgroups. No statistical significance between groups as tested by analysis of variance with Tukey's multiple comparison test. (C) Bacterial counts in spleen homogenates are expressed as logarithmic transformed (log10) CFU per milliliter. WT/WT: n = 21, WT/KO: n = 33, KO/WT: n = 25, and KO/KO: n = 38. (D) Lipocalin-2 concentrations in BALF from 5 randomly selected mice from each of the 4 transplanted subgroups 24 hours after inoculation analyzed by enzyme-linked immunosorbent assay. (E) Lipocalin-2 concentrations in plasma from the same mice analyzed in (D). Horizontal lines indicate means. *P < .05, **P < .01, and ***P < .001 by analysis of variance with Tukey's multiple comparison test.
No differences in bronchoalveolar lavage fluid (BALF) colony forming units (CFUs) were seen among chimeric subgroups (Figure 1B), whereas pronounced differences in the ability to prevent systemic spread of bacteria was observed as demonstrated by an almost 1000-fold higher CFU count from spleen homogenates of KO/KO mice than from WT/WT mice (Figure 1C). The presence of lipocalin-2 in just 1 of the 2 cellular compartments, liver/epithelial cells or myeloid cells, respectively, significantly reduced systemic dissemination compared with KO/KO mice. Interestingly, the contribution of lipocalin-2 from neutrophils/macrophages (KO/WT mice) appeared equivalent to the contribution from the epithelium/liver (WT/KO mice) in terms of resistance to dissemination of K pneumoniae (Figure 1C). The concentration of lipocalin-2 in BALF was comparable between WT/KO and KO/WT mice (Figure 1D) in contrast to the difference seen in plasma (Figure 1E).
The plasma level of lipocalin-2 at 24 hours postinoculation was very low in KO/WT mice (Figure 1E), indicating both that bone marrow–derived cells do not contribute to the rise in plasma lipocalin-2 and that plasma lipocalin-2 is insignificant in protecting against dissemination of infection, because comparable amounts of bacteria were found in spleens from WT/KO and KO/WT mice (Figure 1C) despite much higher plasma lipocalin-2 levels in the former group (Figure 1E).
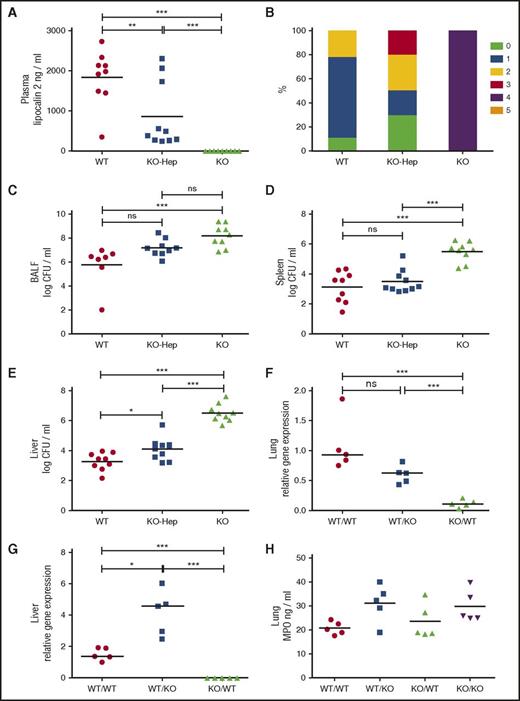
To address the role of liver-derived lipocalin-2, Lcn2 liver-specific knockout (KO-Hep) mice were similarly challenged by K pneumoniae. The level of lipocalin-2 in plasma was significantly lower in KO-Hep mice compared with WT mice, demonstrating that the liver produces the dominating proportion of plasma lipocalin-2 during infection (Figure 2A). Lipocalin-2 total knockout (KO) mice showed a more severe clinical phenotype compared with WT mice, and the KO-Hep mice had scores between these 2 groups (Figure 2B). No statistically significant differences in BALF CFU counts were seen between KO-Hep mice and WT or KO mice, respectively, whereas BALF CFU counts of KO mice were 200-fold higher compared with WT mice (Figure 2C). The latter finding contrasts with our findings on transplanted WT/WT and KO/KO mice, where no difference was seen, and may reflect that lung macrophages in the transplant model are derived from monocytes, and thus might have different immunological efficacy compared with native alveolar macrophages. The total lack of lipocalin-2 in KO mice led to increased dissemination of infection compared with WT mice, whereas the absence of only liver-derived lipocalin-2 (KO-Hep mice) had no implications for dissemination of infection to the spleen (Figure 2D). However, the higher liver CFU counts in KO-Hep mice compared with WT mice suggests a local bacteriostatic effect by liver-derived lipocalin-2 (Figure 2E). In contrast to our findings, a systemic bacteriostatic role for liver-derived lipocalin-2 was suggested in a peritonitis model examining K pneumoniae injected intraperitoneally.9 The contrasting results may reflect that bacteria administered intraperitoneally naturally drains primarily to the liver and thus circumvents the epithelial barriers that are the first line of defense against bacteria delivered by inhalation. The low degree of bacteremia by disseminating K pneumoniae in our model would probably not result in secretion of siderophores to a level at which chelation of siderophores by plasma lipocalin-2 affects the growth of bacteria.
Liver-derived lipocalin-2 is dispensable for protection against dissemination of infection to the spleen, and lipocalin-2 deficiency does not impair neutrophil influx to the site of infection. (A) Lipocalin-2 concentrations in plasma from WT mice, KO-Hep, and KO mice analyzed by enzyme-linked immunosorbent assay. All mice included in the experiment with liver-specific lipocalin-2 knockouts are analyzed. (B) Clinical scores for WT, KO-Hep, and KO mice using the same clinical score criteria as in Figure 1. (C) Bacterial counts are expressed as logarithmic transformed (log10) CFUs per milliliter in BALF for each of the 3 subgroups. (D) Bacterial counts in spleen homogenates are expressed as logarithmic transformed (log10) CFUs per milliliter. (E) Bacterial counts in liver homogenates are expressed as logarithmic transformed (log10) CFUs per milliliter. WT: n = 9, KO-Hep: n = 10, and KO: n = 9. (F) Relative expression of Lcn2 messenger RNA in lung homogenates and (G) liver homogenates from the same 5 randomly selected mice as in Figure 1D-E analyzed by qRT-PCR. No qRT-PCR signal for KO/KO mice was obtained, and so this group was excluded in the statistical testing, which was performed using ∆Ct values. (H) Concentrations of MPO in lung homogenates from the same 5 randomly selected mice from each transplanted subgroup as in Figure 1D-E and panels F-G analyzed by enzyme-linked immunosorbent assay as a measure of the presence of neutrophils in lung tissue. No statistically significant differences between groups as tested by analysis of variance with Tukey's multiple comparison test. Horizontal bars indicate means (A,C-E,H) and medians (F-G): *P < .05, **P < .01, ***P < .001 by analysis of variance with Tukey's multiple comparison test.
Liver-derived lipocalin-2 is dispensable for protection against dissemination of infection to the spleen, and lipocalin-2 deficiency does not impair neutrophil influx to the site of infection. (A) Lipocalin-2 concentrations in plasma from WT mice, KO-Hep, and KO mice analyzed by enzyme-linked immunosorbent assay. All mice included in the experiment with liver-specific lipocalin-2 knockouts are analyzed. (B) Clinical scores for WT, KO-Hep, and KO mice using the same clinical score criteria as in Figure 1. (C) Bacterial counts are expressed as logarithmic transformed (log10) CFUs per milliliter in BALF for each of the 3 subgroups. (D) Bacterial counts in spleen homogenates are expressed as logarithmic transformed (log10) CFUs per milliliter. (E) Bacterial counts in liver homogenates are expressed as logarithmic transformed (log10) CFUs per milliliter. WT: n = 9, KO-Hep: n = 10, and KO: n = 9. (F) Relative expression of Lcn2 messenger RNA in lung homogenates and (G) liver homogenates from the same 5 randomly selected mice as in Figure 1D-E analyzed by qRT-PCR. No qRT-PCR signal for KO/KO mice was obtained, and so this group was excluded in the statistical testing, which was performed using ∆Ct values. (H) Concentrations of MPO in lung homogenates from the same 5 randomly selected mice from each transplanted subgroup as in Figure 1D-E and panels F-G analyzed by enzyme-linked immunosorbent assay as a measure of the presence of neutrophils in lung tissue. No statistically significant differences between groups as tested by analysis of variance with Tukey's multiple comparison test. Horizontal bars indicate means (A,C-E,H) and medians (F-G): *P < .05, **P < .01, ***P < .001 by analysis of variance with Tukey's multiple comparison test.
Lung Lcn2 expression was determined in the 4 transplant groups (Figure 2F). Because lipocalin-2 in neutrophils is synthesized exclusively in neutrophil precursors of the bone marrow and not in mature polymorphonuclear leukocytes,3,11 only Lcn2 transcripts produced by epithelial cells and macrophages are measured by quantitative real-time polymerase chain reaction (qRT-PCR) at 7 weeks posttransplantation. As anticipated, Lcn2 expression in lungs was higher in mice with a WT background compared with a KO background, but no difference was seen regardless of whether lung macrophages were induced to express Lcn2 (Figure 2F, WT/WT vs WT/KO), indicating that the contribution from reconstituting macrophages is small. In the liver, no expression of Lcn2 was observed in KO/KO mice, and only a limited expression, possibly from macrophages, was seen in KO/WT mice (Figure 2G). In WT/KO mice, a higher expression of Lcn2 was evident compared with WT/WT mice, which is consistent with the higher spleen CFU counts and more severe clinical phenotype in the former subgroup.
It has been suggested that lipocalin-2–deficient neutrophils have impaired chemotaxis.12 We determined the influx of WT and lipocalin-2–deficient neutrophils into the lungs by quantifying the amounts of myeloperoxidase (MPO), a protein mainly expressed in neutrophils and, to a lesser extent, in monocytes,13 in lung homogenates from mice challenged by K pneumoniae. Similar amounts of MPO were detected in all 4 groups (Figure 2H). Pathology scores regarding the number of neutrophils confirmed this (supplemental Figure 3D; supplemental Table 1), demonstrating that the presence or absence of lipocalin-2 in neutrophils does not affect their ability to accumulate at sites of infection in vivo at 24 hours postinoculation. No compensatory mechanism reflected by differences in plasma levels of granulocyte-CSF, TNF-α, the chemokines KC and MIP-2, or CXCL5 in BALF was observed (supplemental Figure 4) which, taken together, speaks in favor of neutrophil migration being independent of the neutrophil content of lipocalin-2, a notion that is also supported by others.6,14
Pathology scores based on hematoxylin and eosin staining of infected lungs (supplemental Figure 3D) did not reflect the differences between the 4 transplant groups in the same manner as the clinical scores and spleen CFU counts. The bacteriostatic effect of lipocalin-2 might become more apparent with time because higher pathology scores were observed at 72 hours postinoculation in lungs of Lcn2−/− mice compared with WT mice in a K pneumoniae mouse model comparable to ours.15 However, the 24-hour observation time we chose ensured that there would be a significant clinical effect of the infection without the mice succumbing to the bacterial challenge, because this is ethically unacceptable and would preclude the histopathological analysis.
In summary, we found that lipocalin-2 from both neutrophils and local epithelium is required for optimal resistance against dissemination of infection with a siderophore-producing pathogen. We found that equal protection was provided by lipocalin-2 in WT/KO and KO/WT mice despite higher plasma levels of lipocalin-2 in WT/KO mice. A considerable amount of plasma lipocalin-2 is constituted by liver-derived lipocalin-2, which we find dispensable for protection against dissemination of infection to the spleen. Lastly, lipocalin-2 deficiency does not impair neutrophil influx to the site of infection.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Pia Pedersen, Lars Christophersen, and Dina Silke Malling Damlund for technical assistance, as well as Merete Kjær Hansen for statistical advice.
This work was supported by Sundhed og Sygdom | Det Frie Forskningsråd (11-104328).
Contribution: E.P.C., J.B.C., and N.B. conceived and designed the experiments; E.P.C., S.L.D., K.J.K., and K.T. performed experiments; E.P.C., S.L.D., B.R., C.M., J.B.C., and N.B. analyzed the data; E.P.C. drafted the manuscript; and S.L.D., J.B.C., and N.B. contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.R. is Department of Laboratory Medicine, Karolinska Institutet, Stockholm, Sweden.
Niels Borregaard died on 10 January 2017.
Correspondence: Jack B. Cowland, Coppenhagen University Hospital, 9 Blegdamsvej, 2100 Copenhagen, Denmark; e-mail: jack.cowland@regionh.dk.
References
Author notes
J.B.C. and N.B. are joint senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal