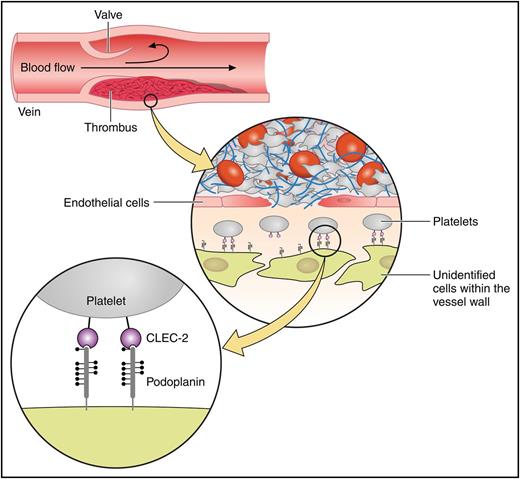
In this issue of Blood, Payne et al revealed a novel function of the platelet C–type lectin-like receptor 2 (CLEC-2)/podoplanin interaction in thromboinflammation: podoplanin expression is upregulated in the vessel wall during venous thrombosis. Venous thrombosis is prevented in the absence of CLEC-2.1 The role of platelet CLEC-2 in thromboinflammation has been previously unrecognized, because CLEC-2 plays a relatively minor role in arterial thrombosis and only a minimal role in normal hemostasis. Moreover, a CLEC-2 ligand, podoplanin is not expressed in the normal vessel wall.
Model showing that podoplanin expression is upregulated in the vessel wall during deep vein thrombosis and stimulates platelets by binding to CLEC-2. Professional illustration by Patrick Lane, ScEYEnce Studios.
Model showing that podoplanin expression is upregulated in the vessel wall during deep vein thrombosis and stimulates platelets by binding to CLEC-2. Professional illustration by Patrick Lane, ScEYEnce Studios.
Suzuki-Inoue et al identified CLEC-2 on the surface of platelets as a receptor for a platelet activating snake venom, rhodocytin/aggretin, and podoplanin as an endogenous ligand of CLEC-2. Podoplanin is expressed on the surface of certain types of cancer cells and various normal cells such as kidney podocytes, type I lung alveolar cells, fibroblastic reticular cells in lymph nodes, and lymphatic endothelial cells (LECs), but not in vascular endothelial cells. Platelet CLEC-2 does not interact with podoplanin under normal conditions, but they interact with each other during pathological conditions or development as embryos. Podoplanin in cancer cells facilitates hematogenous tumor metastasis by binding to platelet CLEC-2, thereby activating the platelets. During embryonic development, the interaction between CLEC-2 and podoplanin in LECs or neuroepithelial cells facilitates blood/lymphatic vessel separation and cerebrovascular patterning/integrity, respectively. After birth, CLEC-2 maintains separation of the lymphatic system from the blood vessels by interacting with podoplanin at the lymphovenous junction. Thus, platelet CLEC-2 plays various roles beyond clotting.2
Although platelet CLEC-2 is highly and specifically expressed in platelets/megakaryocytes, its role in normal hemostasis seems to be minimal, and its role in arterial thrombosis has been controversial. To date, 3 studies have reported that CLEC-2 deficiency in mice does not cause a significant increase in tail bleeding.3-5 CLEC-2 deficiency causes significant inhibition of arterial thrombosis in some experimental settings,3,6 but not in other settings.4,6 Lack of podoplanin expression in the normal vascular wall may explain this phenomena, although it is expressed in the atheromatous plaque in humans and mice.6
Recently, the role of platelet CLEC-2 in maintaining vascular integrity during inflammation has been reported. Boulaftali et al reported that platelets lacking signals from G-protein–coupled receptors, but not CLEC-2–deficient platelets, rescued inflammation-induced hemorrhage in thrombocytopenic mice.7 They hypothesized that platelets leak from hyperpermeable vessels during inflammation and that CLEC-2 signaling is activated by podoplanin, whose expression is induced in the tissue around the vessels or infiltrating macrophages by inflammatory cytokines. Platelet activation by CLEC-2/podoplanin interaction may inhibit hemorrhage through unidentified mechanisms. Herzog et al demonstrated that platelet CLEC-2 also maintains the integrity of high endothelial venules (HEVs) in lymph nodes with the finding that CLEC-2-deficient mice show blood-filled lymph nodes.8 Circulating lymphocytes continuously enter into the lymph nodes from HEVs for immune surveillance, especially during the immune response. Fibroblastic reticular cells around HEVs stimulate platelets by binding to CLEC-2 via podoplanin. Sphingosine-1-phosphate released from activated platelets promotes vascular endothelial cadherin expression on HEVs, which is essential for HEV integrity.
These studies show that inflammation results in leaky blood vessels and may induce podoplanin expression at the site of the inflammations. Payne et al described deep vein thrombosis (DVT) as hypoxia-induced sterile inflammation. Hypoxia develops as a result of flow distortion and stimulates von Willebrand factor release from Weibel-Palade bodies in vascular endothelial cells, which leads to the adhesion of platelets and neutrophils with resultant thrombosis and inflammation. Payne et al found that podoplanin expression is upregulated in the venous wall during this hypoxia-induced sterile inflammation. They demonstrated that the interaction between the upregulated podoplanin and platelet CLEC-2 stimulates venous thrombosis using a DVT model of partial inferior vena cava stenosis with CLEC-2–deficient mice and anti–podoplanin blocking antibody (see figure).
A similar role of CLEC-2 in “unsterile” inflammation has been demonstrated in Salmonella infection–induced thrombosis. In bacterial infection, podoplanin is also upregulated in monocytes accumulating in the liver, which causes liver thrombosis mediated through CLEC-2.9 Shirai et al recently reported extensive thrombus formation in lung in cancer-bearing mice, which was greatly reduced in CLEC-2–depleted mice, which also showed decreased plasma levels of inflammatory cytokines.10 It is tempting to speculate that podoplanin was upregulated in the venous wall during cancer-mediated inflammation.
Thus, CLEC-2 may play a major role in thromboinflammation but a relatively minor role in arterial thrombosis and only a minimal role in normal hemostasis. Thromboinflammation takes a relatively long time to develop, whereas arterial thrombosis and normal hemostasis develop within minutes. Long-term exposure of any inflammatory cytokines may induce podoplanin expression at the site of inflammation.
CLEC-2 may be a good target for the treatment or prevention of thromboinflammation, because CLEC-2 deficiency does not cause significant bleeding tendency. However, a number of issues remain to be solved. For example, what types of cells in the vessel wall upregulate podoplanin expression? What kinds of stimuli under inflammatory conditions upregulate podoplanin expression in the vessel wall? How does platelet CLEC-2 interact with podoplanin expressed inside the vessel wall? Are there any stimulators of platelet CLEC-2 other than podoplanin? Further studies are required to resolve these issues.
Conflict-of-interest disclosure: The author declares no competing financial interests.