In this issue of Blood, Vono et al provide solid data demonstrating that human mature neutrophils can function as antigen-presenting cells (APCs) for memory T cells in vitro.1 Using a rhesus macaque model of vaccination, the authors further demonstrate that antigen-positive neutrophils are abundantly present in draining lymph nodes (dLNs) and spleen, and are fully competent in stimulating the proliferation of autologous antigen-specific memory T cells ex vivo.
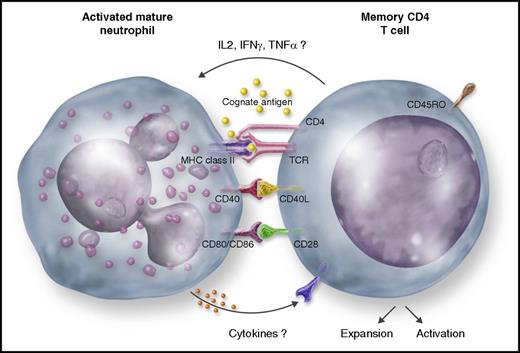
An activated mature neutrophil presenting a cognate antigen to a memory CD4 T cell in a MHC-II–dependent fashion, which, in turn, proliferates, becomes activated, and produces cytokines (IL-2, IFNγ, and TNFα). Likely, costimulator molecules (CD40, CD80, and CD86) play a role in this process. Presumably, CD4 T-cell–derived cytokines activate the neutrophil, whereas neutrophil-derived mediators (cytokines) activate the CD4 T cell.
An activated mature neutrophil presenting a cognate antigen to a memory CD4 T cell in a MHC-II–dependent fashion, which, in turn, proliferates, becomes activated, and produces cytokines (IL-2, IFNγ, and TNFα). Likely, costimulator molecules (CD40, CD80, and CD86) play a role in this process. Presumably, CD4 T-cell–derived cytokines activate the neutrophil, whereas neutrophil-derived mediators (cytokines) activate the CD4 T cell.
The last decades have witnessed a series of remarkable discoveries illustrating that neutrophils, besides acting at the first line of defense against invading pathogens, are highly versatile and plastic cells involved in a variety of physiopathological processes.2 Among various findings, it has been uncovered that neutrophils prolong their survival during inflammation and act as active players of inflammation resolution, display synthetic capacity and function as source of cytokines, play essential roles in regulating angiogenesis and wound healing, exhibit phenotypic and functional heterogeneity, engage crosstalk with leukocyte subpopulations, and link the innate and adaptive arms of the immune response under various circumstances.2-4
In line with this trend, Vono et al report that human mature neutrophils, freshly isolated from seroposive donors with detectable cytomegalovirus pp65-specific and/or influenza hemagglutinin-specific CD4 T-cell recall responses, become able to present the cognate antigens to autologous, antigen-specific memory CD4 T cells, in a major histocompatibility complex class II (MHC-II) (HLA-DR)-dependent fashion. As a result, memory CD4 T cells start to proliferate and produce interferon γ (IFNγ), interleukin 2 (IL-2), and tumor necrosis factor α (TNFα) (see figure). The authors highlight that freshly isolated mature neutrophils constitutively express neither HLA-DR nor costimulatory molecules (including CD40L, CD80, CD86, and CD83), but upregulate all of these molecules once cocultured with the antigen-specific T cells in the presence of the cognate antigen. Consistent with the requirement of both the cognate antigen and responding antigen-specific CD4 T cells, mature neutrophils were found unable to drive a mixed lymphocyte reaction of naive T cells. Notably, all key findings were recapitulated using a rhesus macaque model of immunization with recombinant HIV-1 envelope glycoprotein (Env). Accordingly, neutrophils sorted from vaccine-draining lymph nodes (LNs), in which they abundantly localize, showed expression of HLA-DR and, upon coculture, presented Env to autologous antigen-specific memory CD4 T cells ex vivo. Therefore, according to the criteria recently proposed by Kambayashi and Laufer,5 and based on these current and previous findings (reviewed in Takashima and Yao6 ), human mature neutrophils could be undoubtedly assigned to the club of the so-called “atypical” APCs, which also enlist mast cells, basophils, eosinophils, and innate lymphoid cells.5 Atypical APCs differ from the “professional” APCs, classically represented by macrophages, B cells, and, more effectively, mature dendritic cells (DCs), for their inability to activate naive T lymphocytes. Professional APCs, instead, activate naive T lymphocytes, given their constitutive expression of high levels of MHC-II structural and associated proteins, costimulatory molecules, and a variety of pattern recognition receptors that trigger their activation in response to pathogens.5
Although the notion that neutrophils, either activated in vitro or isolated from various diseases, may express MHC-II and costimulatory molecules, or that neutrophils may function as APCs under certain physiological and pathological conditions, is not novel,6 the study by Vono et al provides convincing control experiments strongly supporting a genuine antigen-presenting capacity by human mature neutrophils. First of all, in addition to ad hoc experiments, the authors use populations of highly purified (about 99%) and viable mature neutrophils, minimizing any possible artifact caused by eventual contaminating professional APCs.7 Then, the authors elegantly performed a side-by-side comparison of the antigen presentation capacity among mature neutrophils and autologous DC and monocyte subpopulations, isolated from either the blood of human donors or the LNs and spleen of immunized rhesus macaques. As expected, neutrophils resulted in the least efficient, but still valid, APCs. In vivo, however, neutrophils, as well as monocytes, corresponded to the most numerous Env+-cell type readily detectable at the muscle injection site and in the dLNs of the arm of immunized rhesus macaques, suggesting that, with their high number, they might overcome their poor antigen-presenting capacity compared with professional APCs.
All in all, the study by Vono et al adds convincing experimental evidence that reinforce the view of neutrophils as cells also involved in adaptive immune responses. Nonetheless, further studies are necessary to definitively clarify how important neutrophils are in the real life in functioning as APCs. Moreover, the precise molecular mechanisms whereby the cognate antigen and antigen-specific memory CD4 T cells promote the expression of MHC-II and costimulatory molecules in mature neutrophils need to be elucidated. Obvious candidates are the T-cell–derived cytokines (see figure). Similarly, it is not completely clear how mature neutrophils could activate antigen-specific memory CD4 T cells. Vono et al show that anti-HLA-DR antibodies prevent T-cell proliferation. However, besides the costimulatory molecule-mediated signals, other T-cell–activating factors might be soluble mediators/cytokines8 derived from cognate antigen/T-cell–activated neutrophils (see figure). Another open question is whether all neutrophils or only a specific subpopulation4 bear antigen-presenting capacity. Vono et al’s study appears to be in favor of the former hypothesis, as the authors show that the few mature neutrophils isolated from the low-density fraction of leukocytes (low-density neutrophils), after blood centrifugation of the same donors, functioned as APCs as efficiently as the normal-density mature neutrophils. By contrast the existence of specialized neutrophil subpopulations, exhibiting the hybrid phenotypic and functional characteristics of both neutrophils and DCs, including antigen presentation capacity, however, originating by immature neutrophil precursors, were reported.6,9,10 Altogether, the latter9,10 and other studies6 therefore indicate that different conditions, environments, and mechanisms may drive the generation of a variety of antigen-presenting neutrophil subpopulations. Finally, could all of these findings translate into a clinical application utilizing newly generated, specific neutrophil subpopulations in boosting T-cell responses in infectious disease or tumor patients?10 Again, future research in the field will give us the answer. Clearly, our knowledge on the biology of neutrophils will never end.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal