Key Points
Absence of CLEC-2 abrogates venous thrombosis.
Podoplanin expression is upregulated in the vessel wall and correlates with the degree of thrombosis.
Abstract
Deep vein thrombosis (DVT) with its major complication, pulmonary embolism, is a global health problem. Mechanisms of DVT remain incompletely understood. Platelets play a role in DVT, but the impact of specific platelet receptors remains unclear. Platelet C–type lectin-like receptor 2 (CLEC-2) is known to maintain the physiological state of blood vasculature under inflammatory conditions. DVT is a thromboinflammatory disorder developing largely as sterile inflammation in the vessel wall. We hypothesized therefore that CLEC-2 might play a role in DVT. Here, using a murine DVT model of inferior vena cava (IVC) stenosis, we demonstrate that mice with general inducible deletion of CLEC-2 or platelet-specific deficiency in CLEC-2 are protected against DVT. No phenotype in the complete stasis model was observed. Transfusion of wild-type platelets into platelet-specific CLEC-2 knockout mice restored thrombosis. Deficiency in CLEC-2 as well as inhibition of podoplanin, a ligand of CLEC-2, was associated with reduced platelet accumulation at the IVC wall after 6 hours of stenosis. Podoplanin was expressed in the IVC wall, where it was localized in the vicinity of the abluminal side of the endothelium. The level of podoplanin in the IVC increased after 48 hours of stenosis to a substantially higher extent in mice with a thrombus vs those without a thrombus. Treatment of animals with an anti–podoplanin neutralizing antibody resulted in development of smaller thrombi. Thus, we propose a novel mechanism of DVT, whereby CLEC-2 and upregulation of podoplanin expression in the venous wall trigger thrombus formation.
Introduction
Platelets are responsible for primary hemostasis and are among the first cells arriving to the site of vascular injury. After adhesion to the exposed subendothelial proteins, such as collagen, and capture of von Willebrand factor (VWF), platelets become activated and develop a clot, sealing the damaged vascular wall. Platelet activation is initiated by various stimulatory ligands, such as adenosine 5′-diphosphate and collagen, which trigger a signaling cascade leading to conformational change in the integrin αIIbβ3 (known as inside-out signaling).
One mechanism of platelet activation is through receptors containing an immunoreceptor tyrosine-based activation motif (ITAM) or the structurally related (hem)ITAM. Murine platelets express 1 ITAM- and 1 (hem)ITAM-bearing receptor, glycoprotein VI (GPVI) and platelet C–type lectin-like receptor 2 (CLEC-2), respectively, whereas human platelets have an additional ITAM-containing protein, FcγRIIA. CLEC-2 is a receptor for podoplanin, which is expressed on various cell types, including lymphatic endothelium and tumor cells, but is absent from the blood vascular endothelial cells.1,2
CLEC-2 seems to play only a minimal role in normal hemostasis. One report demonstrated that CLEC-2 deficiency prolonged tail bleeding time,3 whereas others found an insignificant trend or no increase in blood loss.4-6
In addition to their role in hemostasis, platelets participate in a number of nonhemostatic processes. Platelets have been shown to maintain vascular integrity, regulate endothelial permeability, and prevent hemorrhage at sites of inflammation in the vasculature.7-9 Hillgruber et al found that breaching of the endothelial barrier by neutrophils is a central mechanism of hemorrhage under thrombocytopenic conditions.10 Recent studies demonstrated the critical role of GPVI and CLEC-2 in the prevention of hemorrhaging induced by inflammation.11 Thus, platelet ITAM and hem(ITAM) receptors modulate the functional state of endothelium in inflammatory settings.
Deep vein thrombosis (DVT), with its deadly complication pulmonary embolism, designated together as venous thromboembolism (VTE), is a disastrous health problem. Hundreds of thousands of individuals develop VTE in the United States annually and, despite new therapeutic modalities, the overall prevalence of VTE has not substantially decreased within the last 2.5 decades.12,13 In contrast to arterial thrombosis, which is based on platelet accrual at the ruptured atherosclerotic plaque, the thrombosis in veins occurs without visible endothelial denudation or injury.14 One of the central triggering mechanisms of DVT (with the exception of cancer, trauma, or hereditary hypercoagulability-related venous thrombosis) is stagnancy of blood flow in venous valves, a part of the Virchow’s triad.15 Hypoxia, developed as a result of flow distortion, may render the local environment even more prothrombotic. Blood inside valvular pockets has been shown to rapidly become hypoxic at static conditions with pO2 returning to luminal levels when flow is pulsatile.16 Hypoxia induces release of Weibel-Palade bodies (WPB), which are endothelial granules containing factors implemented in inflammation.17 We have recently demonstrated that release of WPB and cell recruitment to the vein endothelium is a crucial step in a murine model of DVT,18 whereas prevention of WPB liberation through the scavenger receptor B1–endothelial nitric oxide synthase pathway19 protects against DVT in mice.20 In fact, DVT develops similarly to sterile inflammation starting with and depending on recruitment of immune cells and platelets to the vessel wall.21 Consequently, venous thrombosis is tightly associated with inflammatory mechanisms and can be described as thromboinflammation.22 It is known that platelet depletion is protective in murine DVT,21 indicating the central role of platelets in this type of thrombosis. Thus, we hypothesize that platelet receptors, regulating functional properties of the vessel wall in inflammation, could play a role in DVT.
In this study, we used a well-established DVT model of inferior vena cava (IVC) stenosis, mimicking blood flow distortion in venous valves, to study the role of CLEC-2 venous thrombosis. In this model, thrombosis is triggered by endothelial activation in the absence of endothelial denudation or visible injury.21,23,24 We demonstrate a critical role of CLEC-2 in DVT with complete protection of inducible knockout mice and partial protection of platelet-specific knockouts. No phenotype in the complete stasis model was observed. The CLEC-2 ligand podoplanin was expressed in the IVC wall and upregulated during thrombosis, and its pharmacological inhibition decreased DVT thrombus size. These results demonstrate that targeting CLEC-2 could be beneficial for prophylaxis of DVT in the absence of a major effect on hemostasis.
Methods
Mice
All animal experimentation was performed after ethical approval from Animal Welfare Ethical Review Body and the UK Home Office (Project Licenses 70/8286 and 40/3745). Mice 7 to 10 weeks old of both genders were used. All mice were on C57BL/6 background. For inducible deletion of CLEC-2 expression, 4- to 5-week-old Clec1bfl/fl × Rosa26+/ERT2cre mice and their Clec1bfl/fl × Rosa26+/+ control littermates were fed with tamoxifen-supplemented diet TAM 400 (400 mg tamoxifen in citrate form per kilogram of diet, Harlan, UK) for 2 weeks, followed by 4 weeks of regular diet as previously described.25 Clec1bfl/flPf4Cre+ mice (platelet-specific CLEC-2 deletion) have been described elsewhere.26 Vav1Cre+pdpnfl/fl (hematopoietic cell-specific knockout) and Tie2Cre+pdpnfl/fl (knocks out podoplanin in cells of endothelial origin) mice were created by crossing pdpnfl/fl animals with Vav1cre+ mice27 and Tie2cre+ mice (strain 008863; Jackson Laboratory), respectively. Podocin-cre pdpnfl/fl mice were created in house by crossing pdpnfl/fl animals with podocincre+ (stock number 008205; Jackson Laboratory) mice.
Flow restriction in the IVC: a model of DVT in mice
The established flow restriction (stenosis) model of DVT was performed as described previously.18 Mice were anesthetized using isoflurane, placed in a supine position, and attached to a mask with a constant flow of anesthetics. An incision was made along the midline of the abdomen, and the guts were exteriorized. Saline was applied to the guts regularly throughout the experiment to prevent drying out. The IVC was gently separated from the aorta. Any side branches were closed using a 7-0 polypropylene suture. To achieve stenosis (partial flow restriction), the IVC was ligated over a 30-gauge needle or “spacer.” The suture was tied over the spacer, and then the spacer was removed. This procedure achieves ∼90% closure of the vessel lumen but does not cause endothelial injury. The peritoneum was then closed using a 6-0 suture, and the skin was stapled back together. After 48 hours, the mice were culled, and the thrombi, if they developed in the IVC, were taken for analysis. Thrombosis prevalence in wild-type (WT) mice in this model is 65% to 100% after 48 hours of IVC stenosis.18,28
Transfusion of WT platelets into Clec1bfl/flPf4-Cre mice
Blood was collected from the retroorbital plexus of WT C57Bl/6 mice, stabilized by sodium citrate, and centrifuged at 100g for 5 minutes to obtain platelet-rich plasma. Red blood cells were sedimented by centrifugation (100g; 3.5 minutes), and the supernatant (platelet-rich plasma) was incubated with prostaglandin I2 (2 μg/mL, 5 minutes, 37°C) and centrifuged (1000g, 5 minutes). A platelet pellet was resuspended in modified Tyrode’s buffer (134 mM NaCl, 2.9 mM KCl, 0.34 mM Na2HPO4⋅12H2O, 12 mM NaHCO3, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 1 mM MgCl2, 5 mM glucose, pH 7.35). After pooling platelets from a number of donor mice, platelets (8 × 108 in 200 μL of buffer) were infused into recipient Clec1bfl/flPf4Cre+ mice through the tail vein. Transfusion of this amount of platelets results in recovery of >50% of circulating platelets.11 Immediately after that, recipient mice were subjected to IVC stenosis, and thrombus formation was checked after 48 hours.
Treatment of mice with anti–podoplanin antibody
In the DVT experiments, WT littermates were injected with anti–podoplanin antibody (Syrian hamster anti-mouse, clone 8.1.1) twice: 24 hours and 30 minutes before surgery (100 μg per mouse and 50 μg per mouse, IV, respectively). In the intravital microscopy experiments, WT littermates received 1 injection of the antibody (100 μg per mouse) immediately before surgery. Control mice received injection of the same dose of immunoglobulin G (IgG). This antibody has been shown to block podoplanin in mice for at least 48 hours at a dose of 100 μg per mouse.31 Specificity of the antibody was proven by abundant staining of podoplanin in kidneys of WT animals and absence of staining in mice with tissue-specific podoplanin deficiency (supplemental Figure 1, available on the Blood Web site).
Intravital microscopy of the IVC and calculation of the area covered with adhered platelets
Mice underwent IVC stenosis and were allowed to recover consciousness. In 6 hours, mice were anesthetized using tribromethanol as described above, and syngeneic washed platelets (2.5% of the total number of circulating platelets) labeled with calcein AM were injected IV. The mouse was placed into the supine position, and the IVC was exposed and covered with a round coverslip. Fluorescent platelets in the IVC were visualized by a 3i VIVO-SDC confocal system with Yokogawa CSU-10 and Photometrics Evolve EMCCD camera on an Olympus BX61WI upright microscope with air objective ×10. Focus was adjusted to the upper focal plane. Platelets were visualized 1 to 2 mm below the suture (in caudal direction) and recorded for at least 1 minute. Fiji/ImageJ32 was used to quantify areas of adhered platelets. Median-based intensity projection from 10 randomly chosen consecutive acquired images (corresponding to 1 second) was used to generate a single (median) projected image. After an automatic set of threshold, total area of adhered platelets was measured by a Fiji incorporated function “Analyze particles.”
Western blotting
IVC were collected from nonoperated mice or from mice after DVT surgery and lysed. DVT thrombi were separated from IVC and lysed. Fifty micrograms of total protein was loaded per lane and separated in sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Protein gels were blotted to PVC membranes in Turbo Blot Transfer system, blocked with 3% bovine serum albumin, and developed using the following primary antibodies: hamster anti-mouse PDPN (eBioscience, clone 8.1.1), mouse antitubulin (Sigma). Densitometry of bands was performed, and the result was normalized to the density of the corresponding tubulin band.
Immunohistochemistry
DVT thrombi including adjacent IVC were collected and fixed in formalin. Tissue was frozen in OCT. Cryosections (10-μm-thick) were blocked in 5% normal goat serum, 1% bovine serum albumin without permeabilization, and stained using primary antibodies: hamster anti-mouse PDPN (eBioscience), rabbit anti-human VWF (DAKO), followed by Alexa-Fluor–labeled secondary antibodies (Molecular Probes). Nuclei were stained by ToPro3. Confocal images were taken by Leica confocal microscope using company software. Scanning of the whole thrombus was performed using Axio Scan.Z1 (Zeiss). Images were evaluated by Fiji/ImageJ.32
Statistics and power calculation
The sample size estimation was performed using G*Power 3.1.9.2.33 Based on our current experience with the model at the University of Birmingham, thrombi prevalence in control mice after 48 hours of IVC stenosis varies between 60% and 100%. Therefore, we assumed the expected difference (effect size) between experimental groups to be at least 0.8, α error probability of 0.1, and the desired power value of 0.80. The calculated sample size for DVT experiments was 15 animals per group.
Nonparametric data (weight and length of thrombi) were compared using the Mann-Whitney test. Parametric results (area covered by platelets in the intravital microscopy of the IVC) were compared using 2-tailed Student t test. Thrombosis prevalence was compared by the Fisher’s exact square method. Difference was considered significant at P < .05.
Results
CLEC-2 exacerbates DVT
To address the impact of CLEC-2 in venous thrombosis, we used a model of IVC stenosis. Forty-eight hours after stenosis application, mice with a postdevelopment inducible loss of CLEC-2 were completely protected from DVT with none of the mutant mice producing a thrombus as compared with 60% of mice in the littermate control group (Figure 1A). This frequency of thrombosis is similar to previous reports using this model and mouse strain (C57BL/6), where it ranges from 60% to 100%. To test the potential role of platelet CLEC-2, we subjected platelet-specific knockout mice and littermate controls to IVC stenosis. Prevalence of thrombi comprised 81% in controls vs 38% in knockouts (P < .05; Figure 1B). Transfusion of WT platelets (to ∼50% of circulating platelets) restored thrombosis, which further supports the importance of platelet CLEC-2 in DVT. No phenotype was observed in the stasis model (data not shown). Thus, CLEC-2, and platelet CLEC-2 in particular, is critically important for DVT development in the model of partial occlusion.
CLEC-2 exacerbates DVT in mice. (A) Clec1bfl/fl × Rosa26+/creERT2 (n = 10) and their Clec1bfl/fl littermates (n = 9) after 2 weeks of tamoxifen diet followed by 4 weeks of normal diet or (B) Clec1bfl/fl Pf4-Cre mice (n = 13) and control littermates (n = 11) were subjected to IVC stenosis for 48 hours. Some of the Clec1bfl/fl Pf4-Cre mice were transfused with 8 × 108 WT platelets prior to surgery. (i-iii) Thrombus weight, thrombus length, and thrombosis prevalence, respectively. Lines in dot plots represent median. Note restoration of thrombosis after transfusion of WT platelets.
CLEC-2 exacerbates DVT in mice. (A) Clec1bfl/fl × Rosa26+/creERT2 (n = 10) and their Clec1bfl/fl littermates (n = 9) after 2 weeks of tamoxifen diet followed by 4 weeks of normal diet or (B) Clec1bfl/fl Pf4-Cre mice (n = 13) and control littermates (n = 11) were subjected to IVC stenosis for 48 hours. Some of the Clec1bfl/fl Pf4-Cre mice were transfused with 8 × 108 WT platelets prior to surgery. (i-iii) Thrombus weight, thrombus length, and thrombosis prevalence, respectively. Lines in dot plots represent median. Note restoration of thrombosis after transfusion of WT platelets.
Mice lacking CLEC-2 have a reduced level of platelet recruitment to the vessel wall
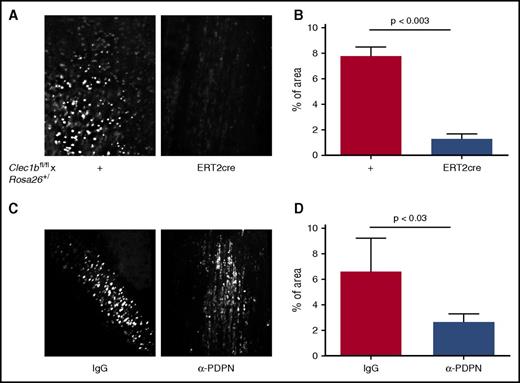
Endothelial activation and resulting accrual of platelets and immune cells in the vicinity of the vessel wall are a central event in DVT development.18,21 We used intravital microscopy of the IVC 6 hours after stenosis application to assess recruitment of platelets. In WT controls, adhered platelets covered the area of 7.8% ± 0.7% of the view field (Figure 2A; supplemental Movie 1). In contrast, in mice lacking CLEC-2, most of the platelets did not attach firmly to the IVC wall and detached in the direction of blood flow (Figure 2A; supplemental Movie 2). The average area covered by adhered platelets in these mice was 1.3% ± 0.4% (Figure 2B; P < .003). Inhibition of podoplanin by a specific neutralizing antibody resulted in a similar phenotype (6.5% ± 1.3% in IgG controls vs 2.6% ± 0.4% after antibody administration; P < .03; Figure 2C-D; supplemental Movies 3 and 4). This result implies that CLEC-2 and podoplanin expression are prerequisites for platelet recruitment possibly through direct binding and by potentiating endothelial activation and WPB release.
Stenosis-induced platelet recruitment is reduced in the absence of CLEC-2 or after neutralization of podoplanin. IVC stenosis was applied to Clec1bfl/fl × Rosa26+/creERT2 and their Clec1bfl/fl littermates for 6 hours. Fluorescently labeled syngeneic platelets were infused, and their deposition on the IVC wall was visualized by intravital microscopy. (A) Representative averaged images of adhered platelets (bright white). (B) Percentage of area covered by immobilized platelets, n = 3 to 4. Data are presented as mean ± standard error of the mean. (C) The same experimental design was applied to WT mice injected with anti–podoplanin neutralizing antibody (100 μg per mouse, IV) or IgG control before surgery. (D) Percentage of area covered by recruited platelets; n = 4 for both groups.
Stenosis-induced platelet recruitment is reduced in the absence of CLEC-2 or after neutralization of podoplanin. IVC stenosis was applied to Clec1bfl/fl × Rosa26+/creERT2 and their Clec1bfl/fl littermates for 6 hours. Fluorescently labeled syngeneic platelets were infused, and their deposition on the IVC wall was visualized by intravital microscopy. (A) Representative averaged images of adhered platelets (bright white). (B) Percentage of area covered by immobilized platelets, n = 3 to 4. Data are presented as mean ± standard error of the mean. (C) The same experimental design was applied to WT mice injected with anti–podoplanin neutralizing antibody (100 μg per mouse, IV) or IgG control before surgery. (D) Percentage of area covered by recruited platelets; n = 4 for both groups.
IVC vessel wall expresses podoplanin
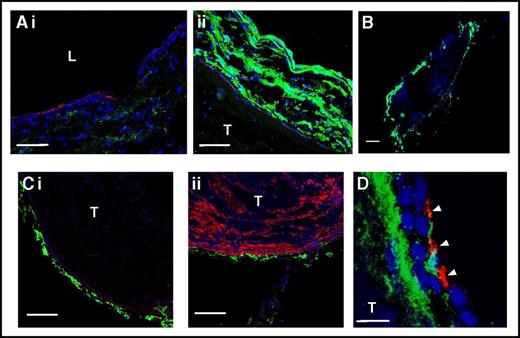
Given that the lack of CLEC-2 and inhibition of podoplanin produce a similar phenotype in terms of cell recruitment, we next asked whether podoplanin is expressed in the IVC wall. Immunostaining revealed a minor podoplanin signal in the wall of sham-operated IVC (Figure 3Ai). Microscopy with identical settings revealed that podoplanin expression in thrombosed IVC was substantially elevated (Figure 3Aii). Podoplanin was expressed exclusively in the vessel wall (not in the thrombus; Figure 3B) and localized in the vicinity of the abluminal side of the endothelium (Figure 3Ci), recognized by positivity for PECAM-1 (CD31). At the microscopy settings used, no podoplanin signal was observed in thrombi, which could be identified by staining for VWF (Figure 3Cii). Platelets penetrated the vessel wall during DVT and had therefore an opportunity to interact with subendothelial matrix components, including podoplanin (Figure 3D).
Podoplanin is expressed in the IVC vessel wall. Sham-operated IVC (Ai) or 48 stenosis-induced thrombi together with the IVC (Aii, B-C) were excised and stained for podoplanin (green) and PECAM-1 (Ai-ii, Ci; red) or VWF (Cii, red). Nuclei are blue in all images. (Ai-ii) Sham-operated IVC and IVC with a thrombus, respectively, photographed under identical microscope settings. Note increased podoplanin expression in the thrombosed IVC. (B) Whole thrombus after 48 hours of stenosis within the IVC. (Ci-ii) Costaining of podoplanin with PECAM-1 and VWF, respectively. Note podoplanin localization below the endothelium and absence of podoplanin in the thrombus. (D) Staining of IVC with a thrombus after 48 hours of stenosis for podoplanin (green) and platelets (CD41, red). Note platelets penetrating the vessel wall and localizing in the vicinity to podoplanin. Arrowheads indicate platelets. (A) Bar, 50 μm; (B) bar, 500 μm; (C) bar, 100 μm; n = 4-5. (D) Bar, 10 μm. Representative images of n = 3 with 10 to 15 images from each IVC. L, lumen; T, thrombus.
Podoplanin is expressed in the IVC vessel wall. Sham-operated IVC (Ai) or 48 stenosis-induced thrombi together with the IVC (Aii, B-C) were excised and stained for podoplanin (green) and PECAM-1 (Ai-ii, Ci; red) or VWF (Cii, red). Nuclei are blue in all images. (Ai-ii) Sham-operated IVC and IVC with a thrombus, respectively, photographed under identical microscope settings. Note increased podoplanin expression in the thrombosed IVC. (B) Whole thrombus after 48 hours of stenosis within the IVC. (Ci-ii) Costaining of podoplanin with PECAM-1 and VWF, respectively. Note podoplanin localization below the endothelium and absence of podoplanin in the thrombus. (D) Staining of IVC with a thrombus after 48 hours of stenosis for podoplanin (green) and platelets (CD41, red). Note platelets penetrating the vessel wall and localizing in the vicinity to podoplanin. Arrowheads indicate platelets. (A) Bar, 50 μm; (B) bar, 500 μm; (C) bar, 100 μm; n = 4-5. (D) Bar, 10 μm. Representative images of n = 3 with 10 to 15 images from each IVC. L, lumen; T, thrombus.
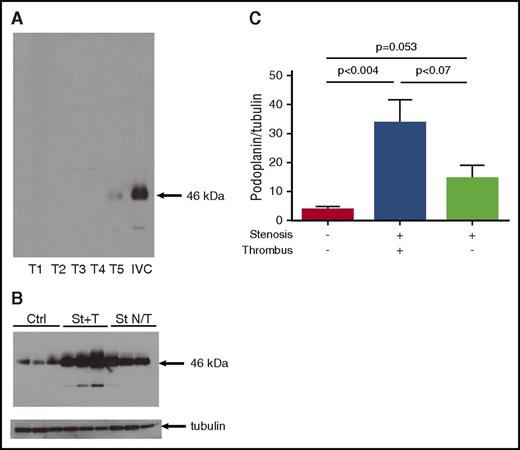
The presence of podoplanin in the vascular wall was confirmed by western blotting. Podoplanin was absent in 4 of 5 thrombi (Figure 4A), with the presence at trace level in the fifth thrombus, probably due to remnants of the vessel wall. Podoplanin was detected in the sham-operated IVC (that had not undergone stenosis) (Figure 4B, 3 left lanes). Application of stenosis for 48 hours, resulting in thrombosis, increased podoplanin expression in the IVC wall from 3.6 ± 1.3 to 33.4 ± 8.3 arbitrary units (a.u.; P < .004; Figure 4C) as measured by band densitometry normalized to corresponding tubulin loading control. Interestingly, application of stenosis for the same period of time but without thrombus formation at the end was accompanied by a much smaller increase in podoplanin expression to 14.3 ± 4.7 a.u. (P = .053 vs intact IVC). Thus, podoplanin expression in the IVC wall is increased during flow restriction, and amplitude of this increase corresponds to the degree of thrombosis.
Expression of podoplanin in the IVC vessel wall increases with thrombosis. (A-C) Thrombi and the IVC were taken separately for western blotting. (A) Western blot of 5 thrombi vs 1 IVC after 48 hours of stenosis. Note complete absence of podoplanin in 4 of 5 thrombi and abundant podoplanin signal in the IVC. (B) Intact IVCs (lanes 1-3), IVCs after stenosis with thrombi (St+T, lanes 4-6), IVC after stenosis without thrombi (St N/T, lanes 7-9). Loading is confirmed by blotting for tubulin. (C) Densitometry of podoplanin bands normalized to tubulin; data in bar graph represent mean ± SD; n = 6 for each group. Ctrl, control.
Expression of podoplanin in the IVC vessel wall increases with thrombosis. (A-C) Thrombi and the IVC were taken separately for western blotting. (A) Western blot of 5 thrombi vs 1 IVC after 48 hours of stenosis. Note complete absence of podoplanin in 4 of 5 thrombi and abundant podoplanin signal in the IVC. (B) Intact IVCs (lanes 1-3), IVCs after stenosis with thrombi (St+T, lanes 4-6), IVC after stenosis without thrombi (St N/T, lanes 7-9). Loading is confirmed by blotting for tubulin. (C) Densitometry of podoplanin bands normalized to tubulin; data in bar graph represent mean ± SD; n = 6 for each group. Ctrl, control.
Anti–podoplanin antibody decreased size of thrombi in DVT
In order to evaluate the role of podoplanin in DVT, we treated mice with an anti–podoplanin blocking antibody (clone 8.1.1).31 The antibody did not affect DVT prevalence (82% in the antibody-treated mice vs 90% in littermate control mice treated with IgG; Figure 5). As no difference was observed in the percentage of mice carrying thrombi, we compared the size of thrombi. Both the weight and the length of thrombi were significantly lower in the group of animals treated with the antibody. This result suggests that CLEC-2 major ligand podoplanin in the vessel wall is implicated in DVT initiation.
Anti–podoplanin antibody decreases size of thrombi in murine DVT. WT mice were injected with neutralizing anti–podoplanin antibody (clone 8.1.1) 24 hours (100 μg per mouse) and 30 minutes (50 μg per mouse) prior to surgery and then subjected to IVC stenosis for 48 hours. Control mice were administered isotype-matched IgG. (A) Thrombus weight, (B) length, and (C) thrombosis prevalence. Lines in dot plots represent median. N = 10 (for IgG) and 11 (for the antibody). NS, not significant.
Anti–podoplanin antibody decreases size of thrombi in murine DVT. WT mice were injected with neutralizing anti–podoplanin antibody (clone 8.1.1) 24 hours (100 μg per mouse) and 30 minutes (50 μg per mouse) prior to surgery and then subjected to IVC stenosis for 48 hours. Control mice were administered isotype-matched IgG. (A) Thrombus weight, (B) length, and (C) thrombosis prevalence. Lines in dot plots represent median. N = 10 (for IgG) and 11 (for the antibody). NS, not significant.
Discussion
Our study demonstrates a pivotal role of CLEC-2 and in particular platelet CLEC-2 in thrombosis. We report that the total lack of CLEC-2 mediates complete protection against DVT, whereas the absence of the receptor specifically on platelets results in partial but significant protection. Inducible knockouts Clec1bfl/fl × Rosa26+/ERT2Cre do not have visible vasculature defects compared with littermate controls (S.P.W., unpublished data). Neutrophils have been reported to express CLEC-2 in 1 study34 but not another.25 The finding that platelet-specific knockout mice have decreased thrombosis suggests a predominant role of CLEC-2 on platelets in DVT initiation. The reason for different median thrombus size in the WT (Cre− littermates) groups of Clec1bfl/fl × Rosa26+/− and Clec1bfl/flPf4Cre+ is unclear. It might be due to different transgenes that these mice carry or tamoxifen treatment or genetic drift due to the lack of cross-breeding between the colonies for years.
It is known that platelet-specific CLEC-2 knockouts have blood-lymphatic mixing, and it is tempting to hypothesize that it may contribute to the lack of thrombosis. However, the postdevelopment inducible knockouts do not have such a defect and have a phenotype even stronger than the platelet-specific knockouts. Also, transfusion of WT platelets into the Clec1bfl/flPf4Cre+ mice restores DVT despite the remaining blood-lymphatic mixture. Thus, it is unlikely that the blood-lymphatic defect affects DVT.
It is known that the functional state of endothelium, a component of Virchow’s triad, is central in the DVT pathogenesis. We previously demonstrated that proinflammatory shift in the endothelium, including release of WPB and recruitment of platelets and leukocytes to the vessel wall, is critical for DVT initiation.18,21 Therefore, mechanisms of DVT initiation and development are similar to aseptic inflammation. Platelets are predominantly recruited through VWF, a WPB constituent.35 Deficiency in CLEC-2 prevents platelet accrual to the IVC wall after several hours of stenosis and before thrombus is formed (Figure 2A-B; supplemental Movies 1 and 2). Reduced platelet recruitment implies that platelets, in a CLEC-2-dependent fashion, directly interact with the vessel wall and that this interaction is required for their recruitment and thrombosis initiation. Stenosis produces elevated blood pressure and formation of a “bulb” with highly distorted flow of low rate. Reduced flow may create local hypoxia, which, similarly to other tissues,36 might upregulate podoplanin expression in the vessel wall. Hypoxia may also render the endothelial cell-cell junctions looser and open, allowing for platelet CLEC-2 interaction with podoplanin and, possibly, with other ligands that are yet to be characterized. Indeed, stenosis facilitates infiltration of the IVC wall with platelets, during which interaction of CLEC-2 with podoplanin or another ligand may take place, promoting thrombus formation. Clarification of the mechanisms of platelet egression to the vascular wall in the course of DVT requires further investigation. Podoplanin upregulation cannot only be a cause for thrombosis but might also be triggered by thrombus formation or both mechanisms may operate in parallel forming a positive feedback.
Interestingly, the anti–thrombotic phenotype was observed only in the partial occlusion (stenosis) model, whereas no difference in thrombosis prevalence and/or thrombus size occurred in the complete stasis model. It is possible that a proinflammatory shift playing a central pathogenetic role in partial occlusion is less important in full stasis in which thrombosis is initiated by tissue factor–driven blood coagulation.37
It has been reported that platelets contribute to maintenance of vascular integrity under inflammatory conditions7 through GPVI and CLEC-2.11 Therefore, CLEC-2 is able to modify functional properties of the endothelium, which might be relevant to DVT development. A similar unique impact of CLEC-2 has been demonstrated in Salmonella infection–induced thrombosis; this is independent of GPVI.38 Marked upregulation of podoplanin in the IVC wall induced by stenosis may promote its interaction with CLEC-2. The importance of the extent of podoplanin expression elevation following stenosis is confirmed by direct association between the magnitude of this elevation and incidence of thrombosis.
Given that DVT can be considered a model of thromboinflammation, the prothrombotic effect of CLEC-2 may be mediated by neutrophils. Flow restriction in the IVC increases expression of various trafficking agents, such as CCL2 and CXCL1, attracting and activating neutrophils.21 Leukocytes are recruited to the IVC wall at the early stages of DVT, and platelets potentiate this recruitment. Neutrophils release neutrophil extracellular traps,39 which provide a surface for activation of coagulation factor XII and form a scaffold in addition to fibrin, thus stabilizing the thrombus.21,40 Neutrophils also release metalloproteinases and other enzymes that can rearrange the vessel wall, rendering it more attractive for platelet recruitment.9 Downregulated platelet accumulation at the stenosed IVC wall in the absence of CLEC-2 could impair leukocyte recruitment and thus contribute to the protection against thrombosis.
DVT can be considered a thromboinflammatory process rather than pure thrombosis. The key role of CLEC-2 in thrombosis in inflammatory settings has recently been reported.38 This study demonstrated that after infection with Salmonella, extensive thrombosis develops in the liver of control mice through an inflammation-driven pathway in a CLEC-2-dependent fashion. Thrombosis induced by the bacteria was associated with upregulation of podoplanin. Both these findings and our results demonstrate the important role of CLEC-2 in thrombosis under either septic or aseptic inflammatory conditions, presumably due to upregulation of podoplanin.
At present, podoplanin is the only known natural ligand for CLEC-2. Its expression has been confirmed in lymphatic endothelium, glomerular podocytes in kidneys, type I alveolar cells in lungs, and in several types of cancers, but not in vascular endothelium.2 However, upregulation of podoplanin in the tissues adjacent to blood vasculature has been reported in the Salmonella-mediated inflammation model,38 and CLEC-2/podoplanin interactions have been shown to secure vessel wall in high endothelial venules.41 We demonstrate here that podoplanin is present in the IVC wall. It is localized immediately below the endothelium and is dramatically upregulated following stenosis of the IVC. This upregulation was substantially higher in those IVCs that had thrombi compared with IVCs, in which, despite stenosis, no thrombi developed. It can be speculated that exposure to podoplanin in the vessel wall triggers platelet accrual and activation, which, in turn, may further upregulate podoplanin expression. This suggestion is further supported by direct observation of reduced platelet accumulation after anti–podoplanin antibody administration. As podoplanin is located at the abluminal side of the endothelium, the mechanism of antibody penetration through endothelial monolayer remains to be elucidated. It is possible that, under the conditions of elevated blood pressure and stretched vessel wall, gaps between endothelial cells become looser, which makes contact between the antibody and podoplanin in the vessel wall possible.42,43 Thus, podoplanin in the venous wall contributes to DVT propagation.
Direct evidence of podoplanin involvement in exacerbation of DVT was obtained in experiments with an anti–podoplanin antibody. Administration of the antibody 8.1.1 resulted in smaller size of thrombi, whereas the percentage of mice producing thrombi remained the same. Thus, the absence of CLEC-2 and inhibition of podoplanin do not produce identical phenotypes in DVT, and it cannot be unambiguously concluded that they operate as a receptor-ligand couple. However, as podoplanin binding to CLEC-2 activates platelets, it cannot be excluded that when podoplanin is neutralized, platelets still interact with the vessel wall through other mechanisms, but their activation is impaired, which results in limited ability to support thrombus growth. Another possibility is that, in addition to podoplanin, another CLEC-2 ligand is involved to mediate complete absence of thrombi observed in CLEC-2-null mice. Incomplete blockage of podoplanin by the antibody in vivo, which precludes downregulation of thrombosis prevalence, also cannot be ruled out.
The source of podoplanin in the IVC remains to be defined. It is unlikely to be hematopoietic or endothelial cell derived as mice lacking podoplanin specifically in these cells retained podoplanin expression in the vessel wall and had no phenotype in DVT (supplemental Figures 2 and 3). The ubiquitous layerlike pattern of staining and the presence of podoplanin in Tie2-Cre pdpnfl/fl mice rule out lymphatic vessels and nodes as a potential source of podoplanin. Fibroblastic reticular cells that surround high endothelial venules have been shown to be rich in podoplanin.41 Podoplanin is also expressed on myofibroblasts of the prostate and cultured normal and cancer-associated fibroblasts.2,44,45 Therefore, it might be supposed that podoplanin in the vein wall originates from cells of fibroblastic nature. Vascular smooth muscle cells (VSMCs) express another potential CLEC-2 ligand, S100A13.46 S100A13 becomes expressed on VSMCs subjected to oxidative stress, which may be present in the IVC as a result of local hypoxia due to stenosis. However, it is not likely that this mechanism is involved in our experiments because venous wall, in contrast to arterial wall, does not contain substantial amounts of VSMCs.
In conclusion, we have demonstrated a major impact of both CLEC-2 and podoplanin in DVT. Total deficiency in CLEC-2 completely protects mice against DVT, whereas the lack of platelet CLEC-2 mediates partial although significant protection. Vein wall expresses podoplanin, and it is implicated in venous thrombosis, although to what extent it operates through CLEC-2 remains to be defined. Given that CLEC-2-null mice have only minimally impaired normal hemostasis, this receptor may be a potential target for DVT prophylaxis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Vyacheslav Kalchenko (Weizmann Institute of Science, Israel) for providing assistance with image analysis and Julie Rayes (University of Birmingham) for performing staining for podoplanin in the kidneys.
This work was supported by the British Heart Foundation (PG/13/60/30406; RG/13/18/30563, CH 3/03) and the University of Birmingham.
Authorship
Contribution: T.P., S.P.W., and A.B. designed the study; H.P., T.P., and A.B. performed research; H.P., T.P., S.P.W., and A.B. analyzed data; and S.P.W. and A.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Brill, Institute of Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom; e-mail: a.brill@bham.ac.uk.
References
Author notes
H.P. and T.P. contributed equally to this work.