Abstract
Target tissue damage occurs as a consequence of pathological immune responses following allogeneic stem cell transplantation resulting in acute graft-versus-host disease (GVHD). Among those who study infections in plants, it is well recognized that tissues play a distinct role from the immune system in mediating disease severity. Recently, this has also been appreciated in mammals. However, the severity of immunopathology in the context of alloimmune diseases such as acute GVHD has been mainly understood and managed by direct targeting of immune cells to generate immune tolerance. The role of tissue-intrinsic factors that might contribute to regulation of acute GVHD severity has been largely overlooked. Here, we introduce the concept of “tissue tolerance” to discuss the tissue-specific programs that contribute to target tissue resilience, repair, and regeneration, and mitigate severity of acute GVHD without altering the load or function of alloreactive immune cells.
Introduction
The immune system is a double-edged sword: although it protects from infection and malignancy, these same inflammatory effector responses can result in destructive immunopathology. This damage can be more than just a side effect; it can be the entire disease itself. A salient example occurs during allogeneic hematopoietic stem cell (HSC) transplantation (HSCT), potentially curative therapy against many hematological diseases, whose utility is crucially limited by the coincident donor immune cell–mediated acute graft-versus-host (GVH) disease (GVHD).1
In order to mitigate its potential for harm, the immune system has evolved mechanisms to self-regulate its response, a concept called immune tolerance. However, limiting acute GVHD through intrinsic and extrinsic immunoregulatory mechanisms without significantly increasing the risks of concomitant infection or relapse has not proven sufficient to mitigate disease at all times. In the setting of infectious disease, a similar contradiction has been observed, where manipulating pathogen burden or immune responses alone is insufficient to maintain host health.2 These observations led to the development of the concept of disease tolerance, a critical property that reduces the pathological impacts of an infection without direct effects on pathogen burden.2-5 Disease tolerance, as seen in plants and animals, is a manifestation of various tissues’ intrinsic, but variable, ability to tolerate or withstand damage from inflammatory immune activity during infection. This implies that both the immune system and tissues are in control of homeostasis during inflammation. We propose that a similar tug of war for homeostatic maintenance also plays out in the setting of noninfectious alloimmune disease such as acute GVHD. We posit that in the setting of acute GVHD, tissue tolerance, or the capacity of a parenchymal tissue to maintain homeostasis in the face of destructive inflammation, could be a crucial player in disease outcome.
In this Perspective, we begin by introducing relatively well-described forms of tolerance, specifically immune and disease tolerance. Next, we outline why we believe invoking tissue tolerance may prove helpful in considering inflammatory disease processes and define the terminology used in our discussion of tissue tolerance. We then summarize the experimental observations that suggest “tissue tolerance” in the context of acute GVHD. We review the data that suggest a role for tissue-inherent properties that may be distinct or work in conjunction with immune responses in the regulation of target organ damage in acute GVHD. We propose an intellectual framework to understand tissue repair, resilience, regeneration, and other aspects under the rubric of “tissue tolerance” in acute GVHD. Although there are as yet no direct and compelling data, we outline the concept of tissue tolerance as a way to generate the hypothesis that can be tested and, if validated, may potentially lead to the development of novel therapeutics and preventative strategies that could potentially be added as adjuncts to immune-suppression strategies.
Current models of tolerance
Tolerance refers to a capacity to endure a given stimulus that would otherwise prove destructive. Current models of tolerance include (a) immune tolerance and (b) disease tolerance.
Immune tolerance
Immunologists are most familiar with the term tolerance as it pertains to immune tolerance, whereby the immune system self-regulates its own inflammatory response (Table 1).1 Immune tolerance could be achieved through several mutually exclusive as well as dependent pathways such as through specialized cell populations (regulatory T cells [Tregs], myeloid-derived suppressor cells), developmental regulation such as negative and positive selection of lymphocytes, or effector-cell–intrinsic properties (activation-induced cell death, T-cell exhaustion).1 However, explaining the severity of immunopathology during infectious inflammation observed in certain clinical and experimental contexts has led researchers to posit an additional mechanism, namely disease tolerance.2-5
Disease tolerance
Disease tolerance and its impact on disease severity was originally appreciated in plant biology where it has been defined as those mechanisms that maintain fitness without directly affecting pathogen or herbivore burden.6-9 Immunologists applied this concept to explain observations in mammalian infection models, where manipulation of immune tolerance and immune resistance, a measure of the immune system’s inflammatory effector response, cannot completely predict disease outcome and impact.2-5 Under this model, a tissue’s inherent ability to withstand or tolerate stress or damage from pathogens, independent of pathogen load, is factored into disease outcome and health2-5 (Table 1).
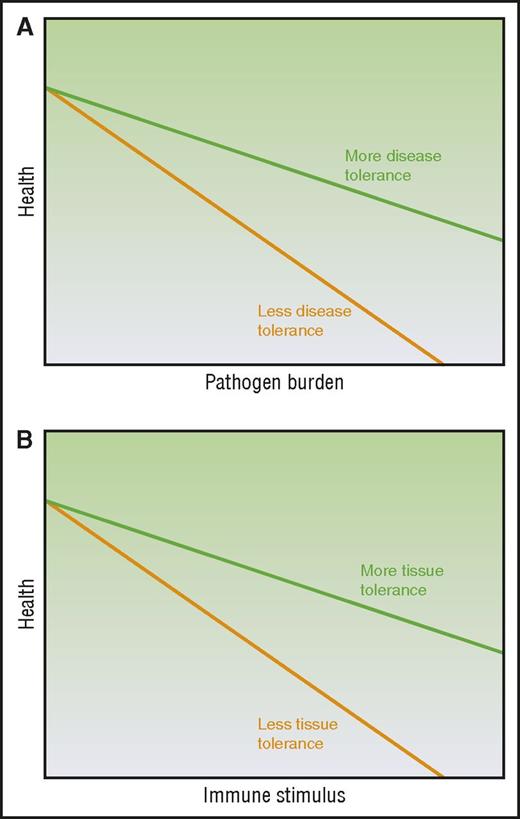
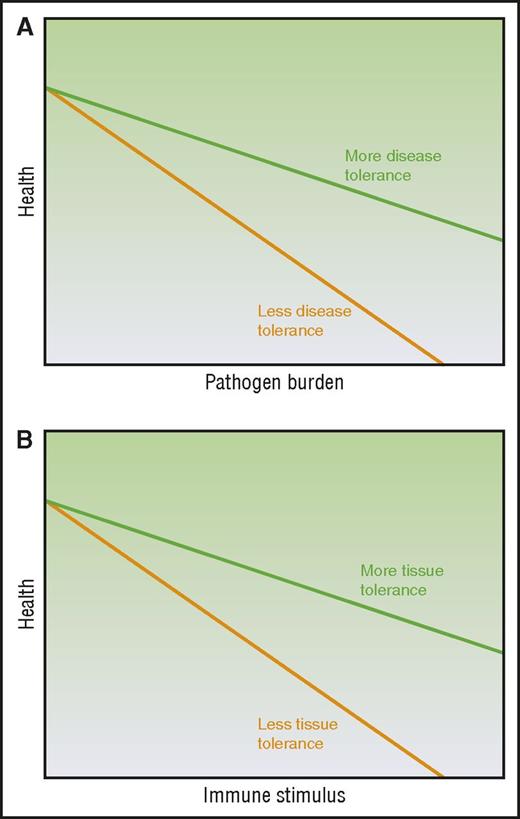
Disease tolerance and immune resistance can be mathematically derived by plotting host health relative to pathogen burden3-5 (Figure 1A), where increasing pathogen burden results in reduced health. The inverse of the pathogen burden represents immune resistance and the slope of the line represents disease tolerance. Therefore, a host with enhanced disease tolerance, where pathogen burden and thus immune resistance has little effect on host health and a better disease outcome, will produce a plot with a line that approaches horizontal (Figure 1A). Evidence suggests disease tolerance is an evolutionarily conserved trait. As reviewed by others,2-5 disease tolerance has been demonstrated in worms,10 insects,11 and vertebrates including mice12-15 and humans.14,16 Although the specific pathways activated in disease tolerance to infection vary from stimuli to stimuli, the hosts demonstrate disease tolerance in response to infection by a diversity of pathogens, from bacteria10-13 to parasites.15
Modeling the relationship between immune elicitor, health, and tolerance. (A) Disease tolerance is measured by plotting host health over changing pathogen burden. (B) Tissue tolerance is determined by plotting host health over changing immune stimuli. Adapted from Ayres and Schneider,3 Medzhitov et al,4 and Schneider and Ayres5 with permission.
Modeling the relationship between immune elicitor, health, and tolerance. (A) Disease tolerance is measured by plotting host health over changing pathogen burden. (B) Tissue tolerance is determined by plotting host health over changing immune stimuli. Adapted from Ayres and Schneider,3 Medzhitov et al,4 and Schneider and Ayres5 with permission.
Tissue tolerance: an expanded model of tolerance in acute GVHD
Our current understanding of the mechanisms driving the immunopathology seen in noninfectious alloimmune settings such as in acute GVHD may be insufficient to fully explain certain observations. Despite the ability to induce massive immunosuppression, we remain unable to completely mitigate immunopathology such as that illustrated by end-stage or steroid-refractory acute GVHD and organ rejection. Furthermore, this is observed in some cases of autoimmunity as well. For example, patients with a genetic immunodeficiency can also show autoimmune symptomology.17 Although it is possible that with even better immune suppression acute GVHD could be mitigated in these contexts, we raise that conjecture that aspects of target tissue resilience, repair, and regeneration that are independent of the alloreactive immune cell burden may be considered as additional mechanisms that can be exploited to mitigate acute GVHD. Therefore, we propose the use of a new concept of tolerance to describe these observations of tissue resilience and repair to damage in the face of sterile alloinflammation: tissue tolerance. We define tissue tolerance as those alloreactive immune cell–independent parenchymal tissue-specific mechanisms that mitigate damage in the face of pathologic acute GVHD (Table 1) and model it as the slope of host health plotted over alloimmune stimulus (Figure 1B). Thus, tissue tolerance might be defined as an improvement in acute GVHD outcome or health and/or fitness for any given burden of alloimmune elicitor/mediator. When interpreting the literature through this lens, we see observations that host parenchymal tissue factors may in fact contribute to disease outcomes in acute GVHD alongside immune tolerance and resistance. A recent publication has also used the term tissue tolerance in the context of infection,18 but here we will confine the use to the noninfectious setting, specifically acute GVHD.
Tissue tolerance and allogeneic HSCT
HSC rejection
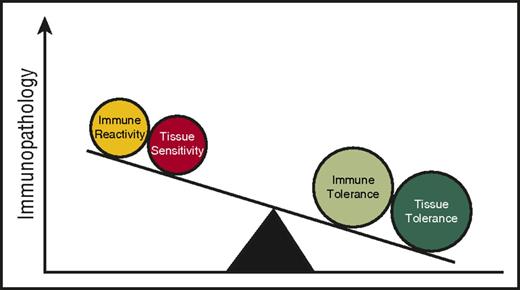
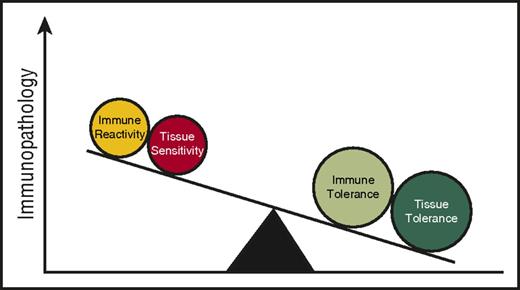
HSC rejection (host versus graft) and GVHD, depending on the direction of immune reactivity, are the potential consequences of an allogeneic immune reaction following allogeneic HSCT. Are there HSC-autonomous features that might explain why HSC rejection occurs in some patients despite stringent immunosuppression after allogeneic HSCT? Some insight may be obtained by examining studies on allogeneic solid organ transplant rejection. Both clinical and experimental observations suggest a role for tissue tolerance in mediating the severity of allograft rejection. For instance, in kidney transplantation, donor and recipient are typically matched by blood type to prevent hyperacute rejection. However, when ABO-mismatched transplants have been performed, not all grafts are universally rejected.19 “Accommodation,” a process that involves graft organ–specific factors and provides protection against immune-mediated rejection has been postulated in this context. Specifically, in “accommodated” organs, complement and donor-reactive antibodies are able to bind but subsequent lysis is reduced.19,20 The specific mechanisms mediating these protective phenotypes are not understood, but the observations can be viewed as target cell/tissue-intrinsic adaptive mechanisms that mediate resistance to the immune-mediated attack of allogeneic organs. Viewed in this light, it could be speculated to be part of the tissue tolerance model. It is important to consider the differences in the clinical context of solid organ vs HSC allografting (for example, the intensity of conditioning, the duration of immunosuppression) and mechanisms may as such vary. Experimental models have yet to evaluate such a notion in HSC rejection following allogeneic HSCT but it may be possible, but not certain, that better insight into the mechanisms governing accommodation will afford better insight into the mechanisms of HSC rejection. Prevention of allogeneic HSC rejection may perhaps be due to the net effect of both immune and tissue tolerance (Figure 2).
Tissue tolerance interacts with immune tolerance to mediate immunopathology.
Acute GVHD
Several observations and emerging experimental data point to a potential role for target tissue–autonomous features in determining the clinical severity and mortality of acute GVHD. These features may be independent of the quantity, intensity, and magnitude of alloreactive T cells or inflammation.
Clinical observations.
Several clinical observations point toward the existence of potentially additional determinants to immune cells and inflammation in causing acute GVHD severity. Although there are no definitive experimental data, we would suggest that the following scenarios may be considered under the rubric of tissue tolerance that might lend themselves for hypothesis generation and could be explored with rigorous experimentation. For instance:
Target organ specificity.
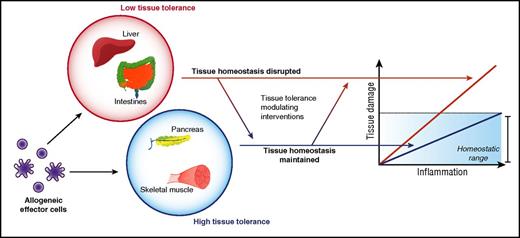
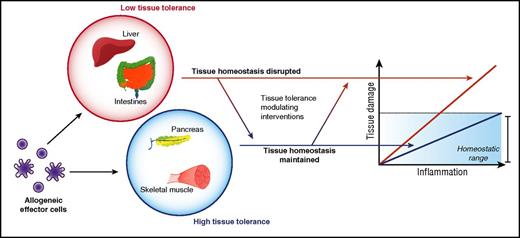
In most clinically significant acute GVHD mediated by alloreactive T cells, immune pathology is largely restricted to the skin, liver, and gastrointestinal (GI) tract. This is despite ubiquitous expression of alloantigens and the ability of donor alloreactive T cells to gain access to many other tissues. In this section, we explore a potential hypothesis that may explain this target organ specificity. The presence of a microfloral interface may explain acute GVHD target organ specificity, but it fails to explain why genitourinary and upper aerodigestive tracts are spared from acute GVHD, whereas the liver remains a bona fide target. Because the GI tract, skin, and liver are large organs, the size of the allogeneic target tissue may be posited as a determinant. However, other large targets, such as skeletal muscle, are spared. But these features are clearly not germane to chronic GVHD. In fact, the limited tissue specificity of acute GVHD, when viewed in the longer time scale after allogeneic HSCT is puzzling given the much varied and additional tissue involvement in chronic GVHD. This could obviously be a consequence of time duration and/or the type of immune mechanisms of acute and chronic GVHD. However, whether and if the tissue tolerance concept can be expanded to chronic GVHD as well remains an intriguing question that is beyond the scope of this manuscript. Similarly, although the clinical context of allograft rejection and acute GVHD are different, the fact that kidney, pancreas, heart, and lung allografts are rejected following solid organ transplant but are largely deemed not to be target organs of acute GVHD indicates that alloantigen expression and alloimmune effector functions are not predictors of disease organ specificity in acute GVHD (Figure 3).
Differential organ tissue-specific responses to allogeneic immune attack.
Therefore, although it is possible that many organs are targeted by an acute T-cell–dependent GVH reaction, often only a subset will experience immune-pathological damage significant enough to give rise to clinical signs and symptoms of disease. This, we would like to speculate, might be because of some other inherent property of parenchymal tissues that modulates tissue susceptibility or tolerance to alloimmune damage in acute GVHD (Figure 3). Thus, it may depend on how a tissue is able to protect itself, that is, “tolerate” an immune-mediated attack by alloreactive T cells, extending an idea proposed by Matzinger, who had hypothesized that tissues may actively modulate immune responses.21,22
Immune-suppression refractory acute GVHD.
When viewed from the perspective of tissue tolerance, steroid (immune-suppression) refractory acute GVHD may prove more explicable. Often understood and treated only as an immune tolerance/reactivity issue, patients who have developed steroid-refractory GVHD are treated with a wide array of extremely potent immune suppressants.23-32 Although the immune system appears massively suppressed from the “clinical” standpoint (manifested by opportunistic infections, cytomegalovirus, Epstein-Barr virus, and other viral reactivations),24-26,28,31,32 many steroid-refractory patients seldom show “clinical” response to such an intense immunosuppression strategy. This suggests that suppressing immunity, at least to the point of the clinical consequence of severe viral reactivations, infections by opportunistic organisms, and relapse, may not be sufficient to reduce mortality and morbidity from steroid-refractory GVHD. However, besides the clinical evidence of massive immune suppression, the exact amount of immune suppression, within the limits of ex vivo assays, has not been studied. It is nonetheless possible that more immune suppression may still be able to mitigate GVHD, but this will have severe clinical consequences. We would like to suggest that in these cases, it may be possible that in addition to (and not instead of) targeting the immune system alone, enhancing target tissue–intrinsic mechanisms that promote tissue tolerance may provide an opportunity to improve outcomes.
Improvement in GVHD outcomes independent of direct targeting of immune system.
Recent improvements in clinical outcomes of acute GVHD despite similar immune-prophylaxis regimens have not only been attributed to better matching, but also to better supportive care.33 Some of the supportive care measures are due to improvements in antibiotics, antivirals, and antifungals. Others are improvements in general supportive care that include better, more appropriate fluid, electrolyte, and nutrition strategies. Many of these measures do not directly alter the load or function of alloreactive T cells or the magnitude of inflammation. Instead, these are likely enhancing the cellular and tissue-adaptive/repair responses and thus limiting the deleterious effects of T-cell– and inflammation-mediated stress and damage.
Mechanisms.
Direct and specific experimental explorations into the concept of tissue tolerance after allogeneic HSCT are lacking, in part, maybe because of lack of consideration of such a concept in acute GVHD. As such, the mechanisms remain unknown. However, some experimental data, when viewed through the lens of the tissue tolerance, may shed light on potential mechanisms. Although the observations summarized in the following sections are all related to intestinal epithelial tissue behavior following allogeneic HSCT, whether similar or distinct mechanisms are used by liver or skin during acute GVHD remains to be determined.
Microbiota and metabolites in acute GVHD.
Previous observations have suggested a role for intestinal microbiota in the pathogenesis and severity of acute GVHD.34 Recent studies have clearly demonstrated a strong correlation between shifts in the intestinal microbiome and acute GVHD severity, both in experimental and human contexts.35-37 Besides characterizing and enumerating the changes in intestinal microflora, recent work has also begun to explore the role of the intestinal metabolome in tissue homeostasis and acute GVHD severity. The microbiota perform key metabolic functions; they not only break down material directly ingested by the host but also produce their own metabolic byproducts.38,39 The intestinal metabolome thus consists of products from discrete host metabolism, microbial metabolism, and mammalian-microbial cometabolism.40 The impact of microbiota-derived metabolites is being increasingly appreciated, specifically in intestinal homeostasis.40 A recent study explored the effects of metabolic byproducts on acute GVHD in a major histocompatibility complex mismatch model of experimental bone marrow transplant (BMT).41 It made the surprising observation that among the short-chain fatty acids, only butyrate, the primary energy source for intestinal cells, was reduced in the intestinal epithelial cells (IECs) isolated from acute GVHD animals.41 The study demonstrated that butyrate supplementation promoted intestinal barrier function in vivo and, ex vivo, enhanced the ability of IECs to survive and withstand alloreactive T-cell and inflammation-induced damage.41 This increased survival was associated with increased expression of junctional proteins promoting GI barrier integrity and increased expression of antiapoptotic proteins, promoting IEC survival.41 Mice treated with butyrate or with butyrate-producing microbes exhibited reduced acute GVHD–associated morbidity and mortality.41 Although Tregs are known to be induced by butyrate,42 the study demonstrated that donor Tregs were not critical for GVHD protection when butyrate was delivered locally, either directly or indirectly, through shifting the microbiome toward high butyrate–producing Clostridial species.41 Furthermore, ex vivo analyses of donor T cells from the spleen showed no systemic effects of butyrate activity and no change in function when compared with nonbutyrate–treated animals with active acute GVHD,41 indicating that the beneficial effects on acute GVHD severity were likely from local trophic effects on IECs, enhancing their ability to “tolerate” immune-mediated damage. These data are not definitive, but suggest that enhancing IEC barrier function and resilience may mitigate acute GVHD without significantly modifying the local alloreactive T-cell burden. In another study, in hosts with host parenchymal target tissue–specific loss of NLRP6 signaling, acute GVHD severity was reduced, again with no significant effect on alloreactive T-cell burden.43
Regeneration of intestinal target tissue.
Experimental evidence suggests regeneration of damaged tissue is another strategy used by tissues to increase tolerance against acute GVHD–associated inflammation, independent of alloreactive T-cell burden. Administration of keratinocyte growth factor, a critical regulator of IEC growth,44 cannot only protect GI tissue from the harmful effects of the conditioning regimens for bone marrow transplant45 but also reduce morbidity and mortality from acute GI GVHD.46,47 However, the studies did not directly explore effects on target tissues and inferred benefits from reshaping the donor immune response.
More recently, studies have directly explored how promoting intestinal stem cell (ISC) survival and repair mitigates acute GI GVHD. Specifically, treatment with Wnt-agonist R-spondin1 to stimulate the Wnt-signaling pathway normally responsible for regulating IEC proliferation not only protects ISCs from damage due to conditioning therapy but also ameliorates acute GVHD pathology under otherwise identical transplant conditions.48 Recent elegant studies with interleukin 22, released by innate lymphoid cells, showed that interleukin 22 primarily exerts its effects on ISCs to reduce acute GVHD severity.49 This protection did not mitigate graft-versus-leukemia responses or alter donor T-cell responses. These data collectively show that it is possible to reduce acute GVHD severity without directly altering the load or function of immune cells but instead by altering the ability of target tissues to directly resist, repair, or regenerate from immunopathology (Figure 3).
Future perspectives
Exploring the mechanisms that promote tissue tolerance and exploiting them for therapeutic benefit in addition to continued understanding of immune tolerance could represent a novel area for research in acute GVHD. Mechanisms of immune and disease tolerance are complex. Similarly, we posit that tissue tolerance pathways may be complex involving >1 mechanism of action. They may be classified into categories, much like those used to organize immune and disease tolerance mechanisms. Although many of the mechanisms of immune tolerance (central and peripheral) and the various distinct and overlapping cellular, signaling, epigenetic, and molecular pathways are known, much remains to be understood. Increasing experimental data41,43,45-49 are starting to illuminate pathways that might be divided into parenchymal tissue cell-intrinsic and -extrinsic pathways of disease tolerance that include increased protein production, elevated concentrations of reactive oxygen species which can lead to DNA damage, and altered nutrient resource pools (reviewed in Soares et al,2 Ayres and Schneider,3 Medzhitov et al,4 and Schneider and Ayres5 ). It is plausible that the mechanisms of tissue tolerance may be similarly linked to tissue-specific adaptations during acute GVHD to mediate tissue homeostasis. Our conjecture is that tolerance mechanisms are likely to be tissue-autonomous features that regulate their repair and regeneration during homeostasis and/or under stress. These pathways may be similar or distinct between the 3 different organs of acute GVHD. It remains unknown whether pathways of immune and tissue tolerance overlap or act antagonistically or synergistically. Evidence from the various salutary effects of butyrate, and the overlapping role of Toll-like receptor signaling in immune and tissue functions point to potential overlap of at least some of the pathways of immune and tissue tolerance.
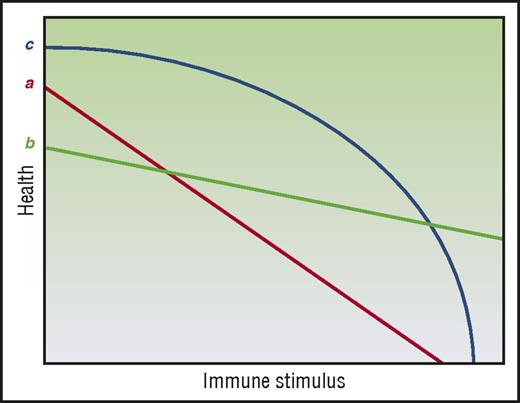
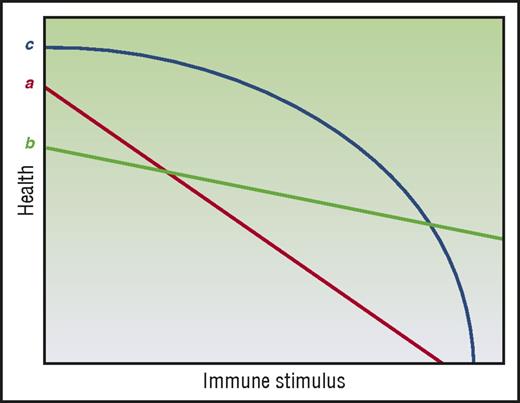
Mechanisms of tissue tolerance may also be observed not just at the cellular level but also at the organ level. Specifically, the ability of an organ tissue to maintain function in spite of pathogenic immune effectors can vary compared with other organ tissues. Interestingly, the acute GVHD target organs, namely, liver, skin, and GI tract are seemingly among the most tolerogenic organs given that (a) they can regenerate and repair injury and (b) that damage to 1 area of the organ does not necessarily compromise the entire organ. Thus, within a certain range, liver and GI damage can be asymptomatic (subclinical acute GVHD). This then presents an apparent contradiction with the fact that liver, skin, and GI tract are most susceptible to alloimmune-mediated acute GVHD. It also offers no clear explanation to the clinical observation that in acute GVHD, GI and skin involvement are seen at almost twice the rate of liver involvement.50 One possible solution may be inferred from the hypothesis laid out by Little et al.51 In considering 2 tissues, a and b (Figure 4), health under low-stress conditions may appear to be greater for a, but, under high-stress conditions, the picture may change and b may show greater tolerance. Under this framework, a tissue’s tolerogenic character cannot be fully described by studying its responses to only 1 category and intensity of stress. Furthermore, tissue tolerance may not be linear, such as in tissue c (Figure 4). In this instance, the intensity of the GVH response, which may be impacted not just by load but also by time, will produce different pictures of tissue tolerance. Thus, mechanisms of tissue tolerance in the context of acute GVHD may be specific to the organs, that is, mechanisms and time scales that improve GI barrier function may not be germane to liver or skin GVHD.
Tissue tolerance is context dependent. (Line a) A linear relationship by health and immune burden regulated by tissue tolerance. (Line b) Another linear relationship, but one where tissue tolerance may appear to be greater or less than (line a) depending on measurement conditions. (Line c) A nonlinear mode of tissue tolerance–mediated regulation of health where tissue tolerance may appear relatively high or low depending on measurement conditions. Adapted from Little et al51 with permission.
Tissue tolerance is context dependent. (Line a) A linear relationship by health and immune burden regulated by tissue tolerance. (Line b) Another linear relationship, but one where tissue tolerance may appear to be greater or less than (line a) depending on measurement conditions. (Line c) A nonlinear mode of tissue tolerance–mediated regulation of health where tissue tolerance may appear relatively high or low depending on measurement conditions. Adapted from Little et al51 with permission.
In conclusion, in all cases after allogeneic HSCT, immunosuppression or attempts at promoting immune tolerance are mandatory to reduce acute GVHD, however, in some cases, it may be relevant to have additional or complementary strategies that also focus on target tissue–intrinsic mechanisms to mitigate severity. Considering tolerance as involving both immune system and the parenchymal/epithelial tissues could lead to generation of novel hypotheses which can be tested. We posit that including “tissue tolerance” into the conceptual tool kit of allogeneic HSCT may allow for better understanding of organ damage from acute GVHD. We emphasize that this will not replace efforts at targeting immune tolerance, but instead may be an additional strategy in some cases of acute GVHD. If this concept is validated, it is possible to consider therapies that increase tissue tolerance to protect organ damage as an adjunct to efforts that enhance immune tolerance.
Acknowledgments
This work was supported by National Institutes of Health grants HL090775 and HL128046 from the National Heart, Lung, and Blood Institute, and CA173878 and CA203542 from the National Cancer Institute.
Authorship
Contribution: S.-R.W. analyzed and wrote the paper; and P.R. conceived, analyzed, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pavan Reddy, University of Michigan Cancer Center, 3215 CCGC, 1500 E Medical Center Dr, Ann Arbor, MI 48109; e-mail: reddypr@umich.edu.